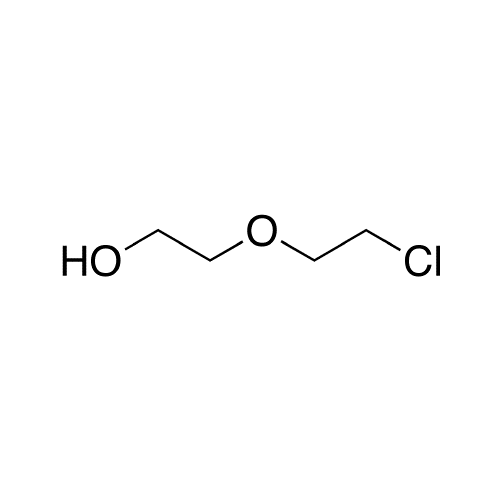
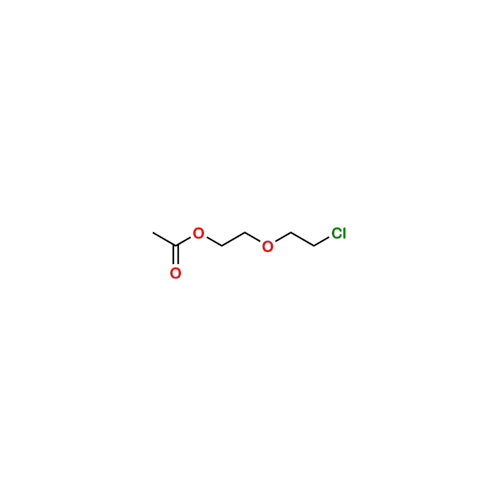
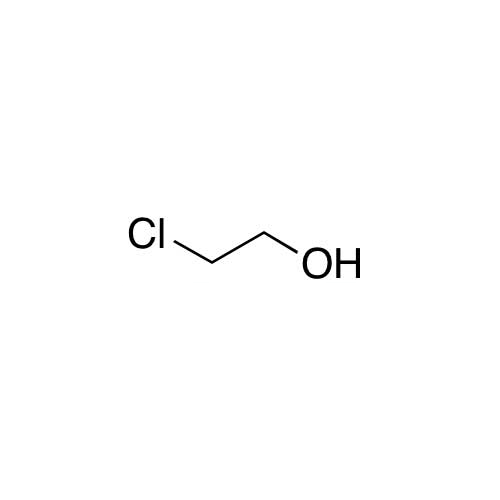
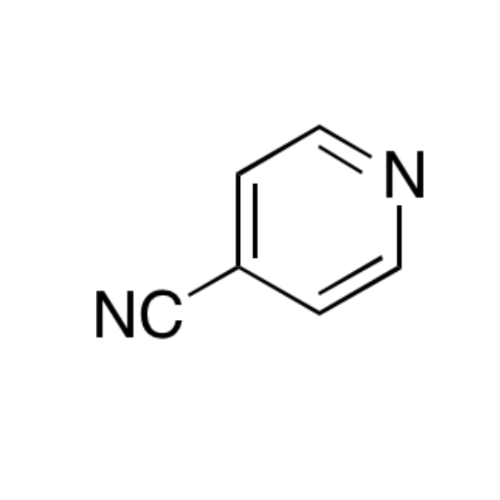
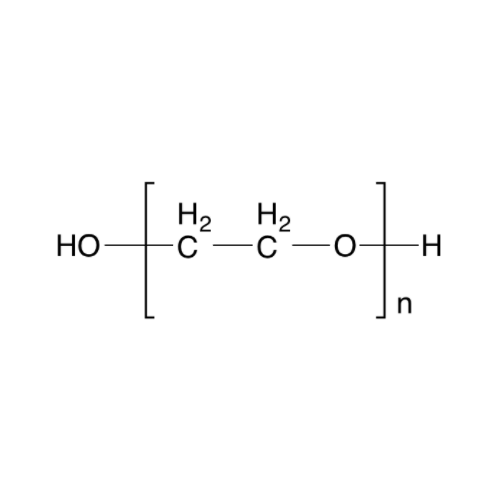
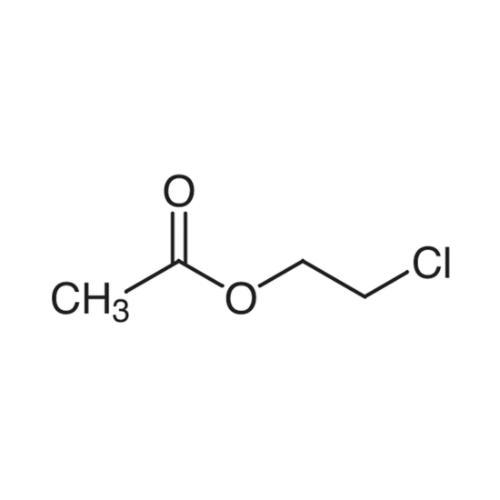
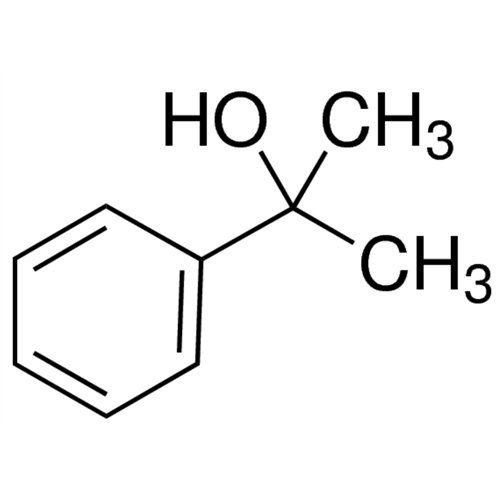
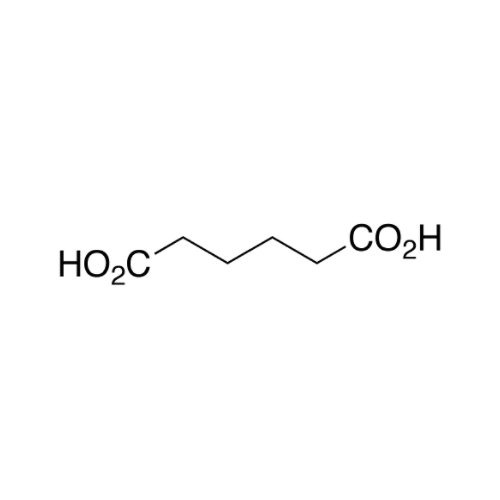
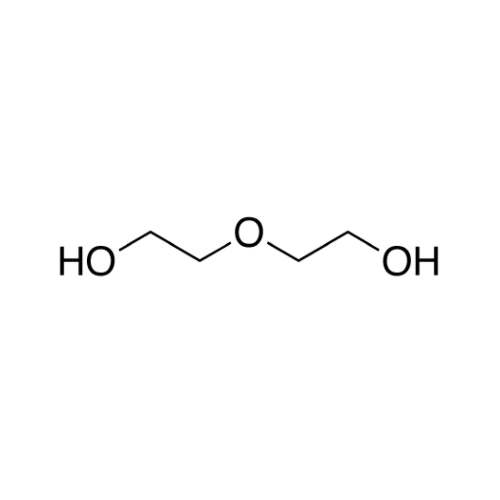
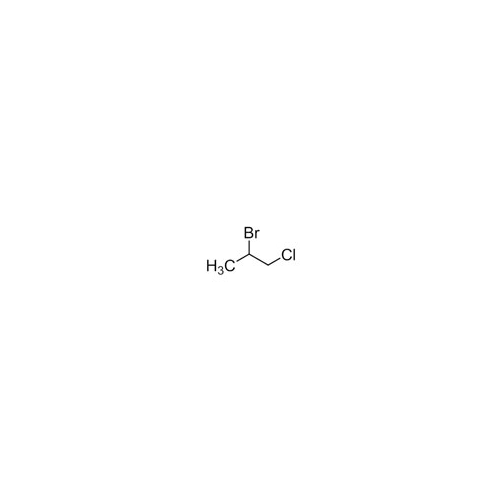
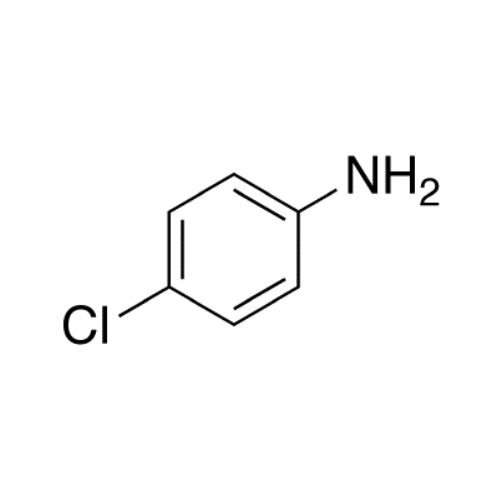
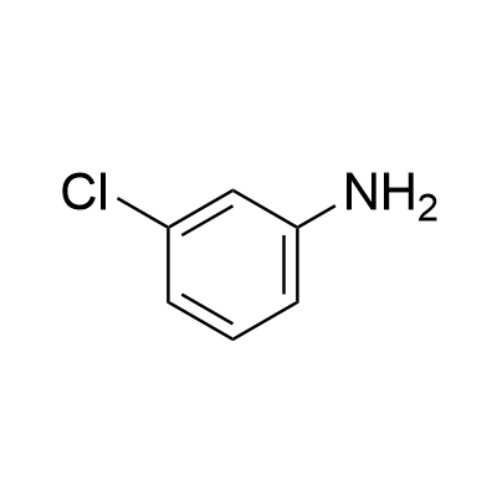
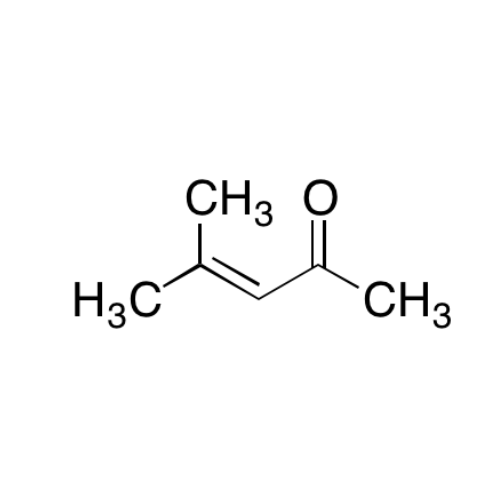
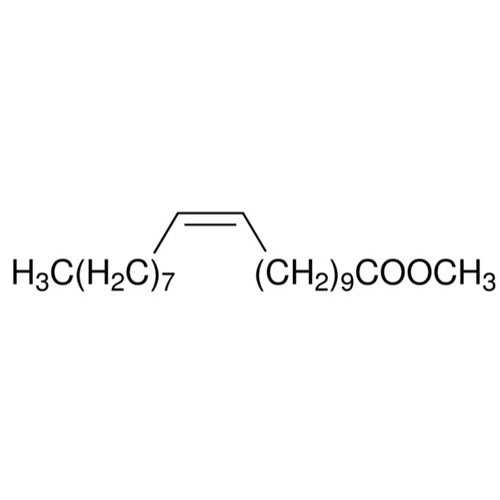
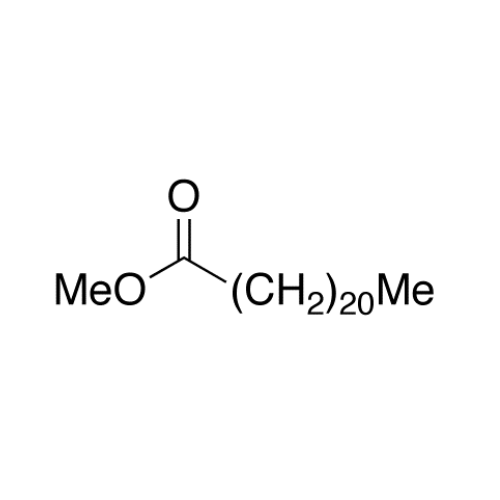
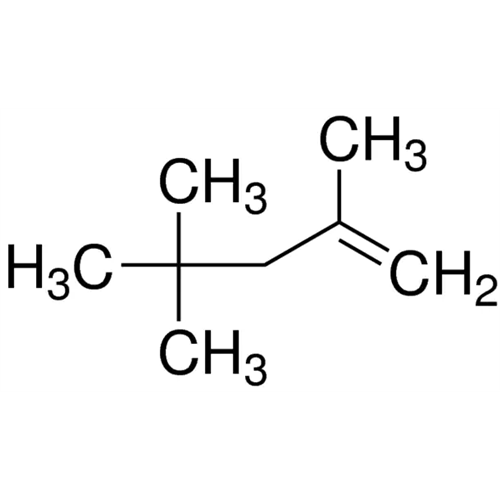
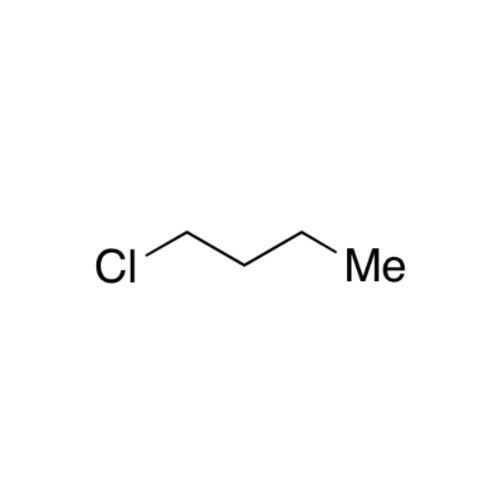
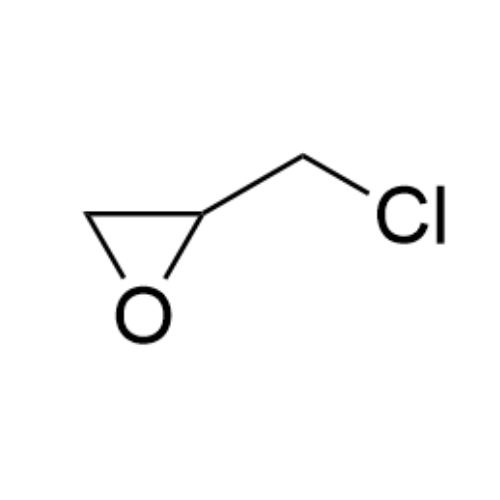
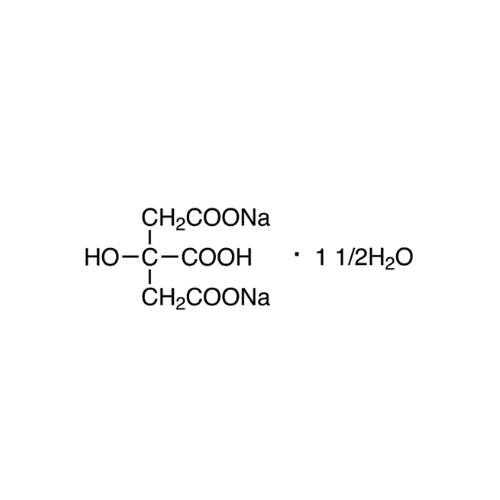
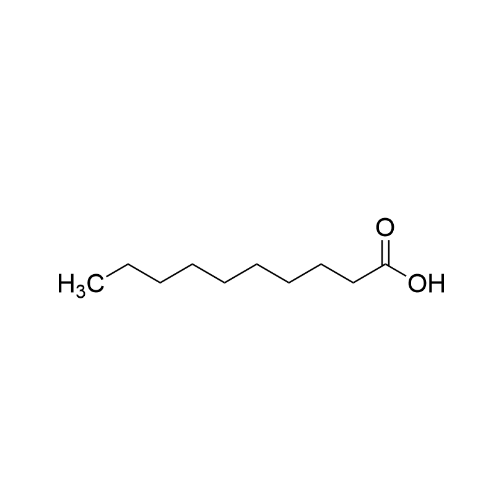
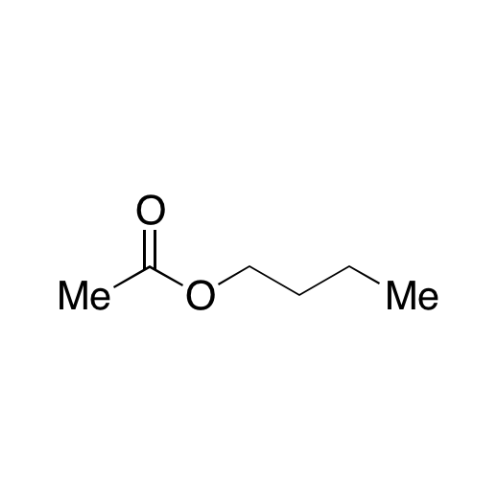
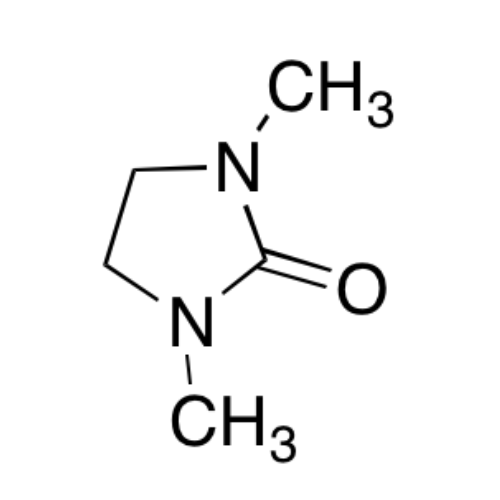
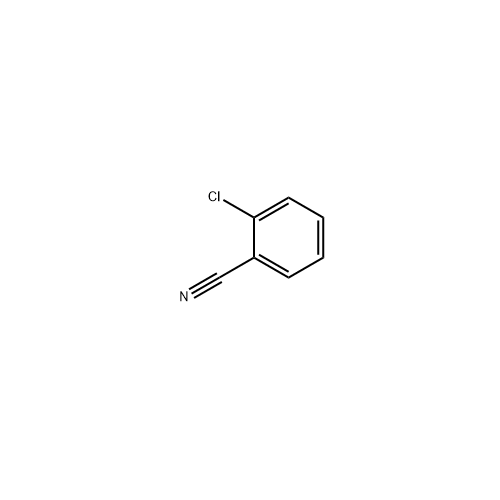
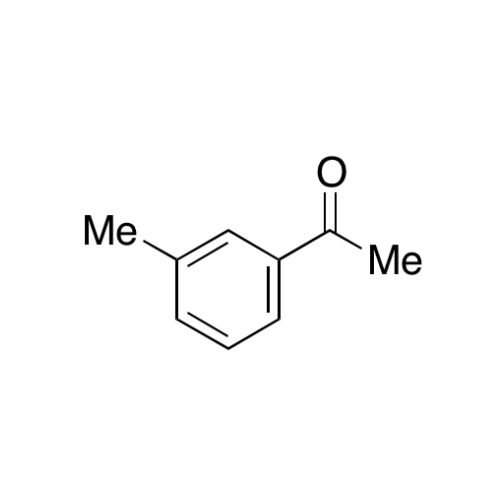
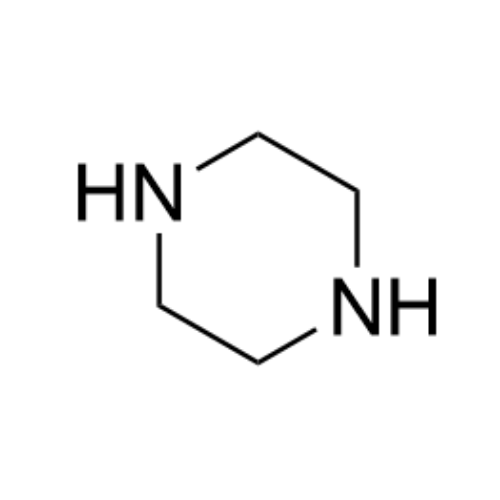
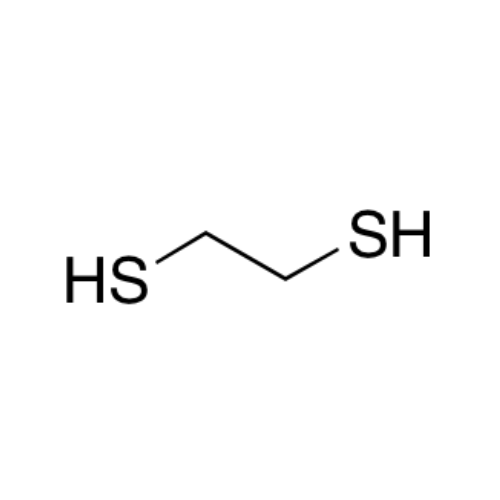
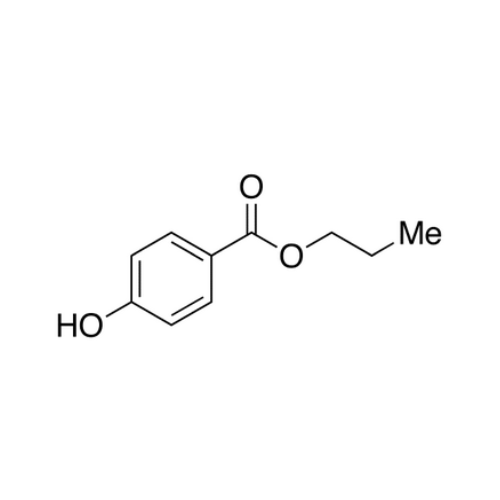
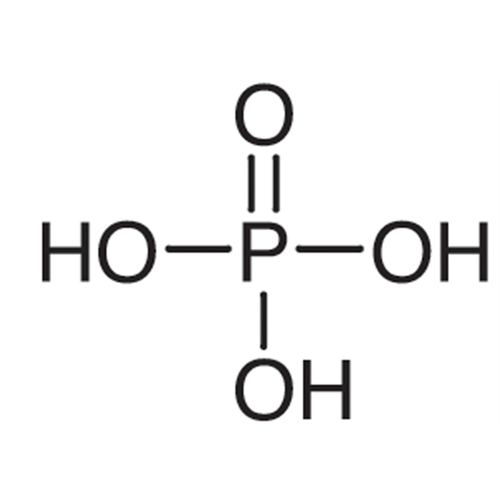
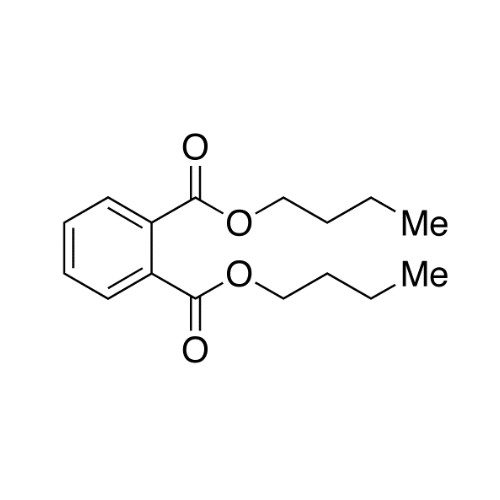
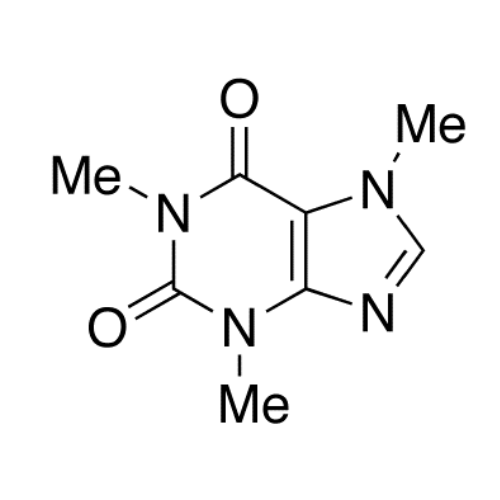
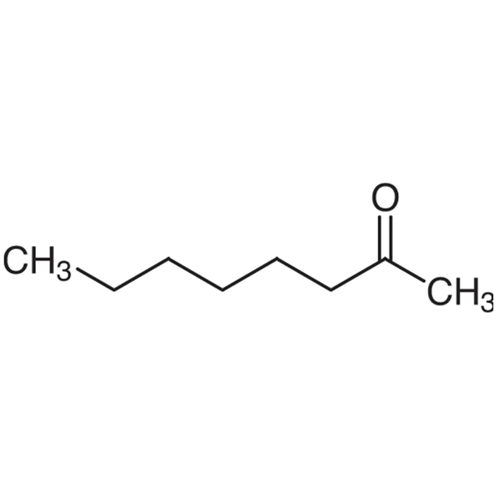
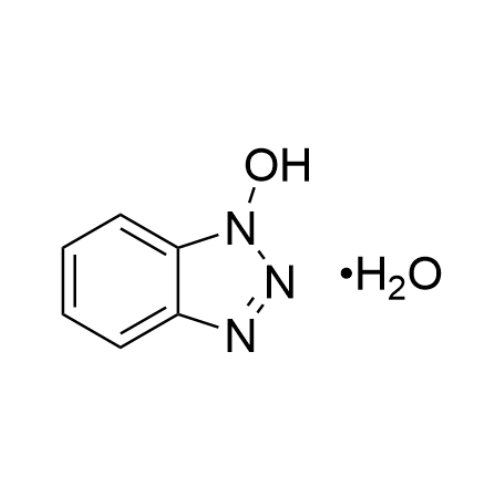
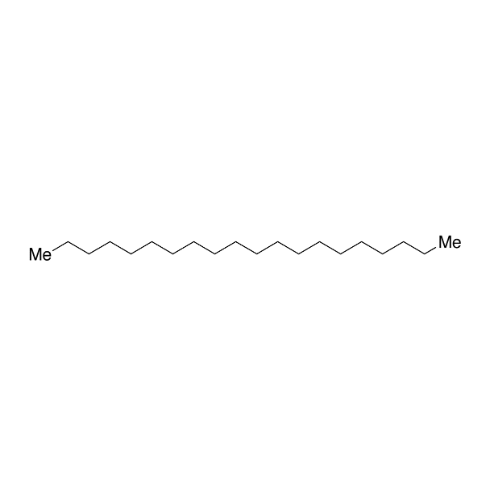
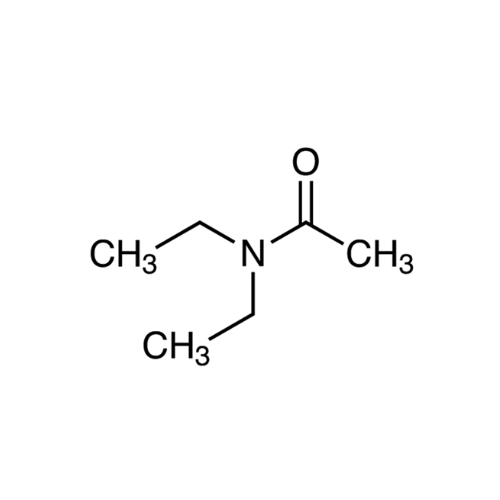
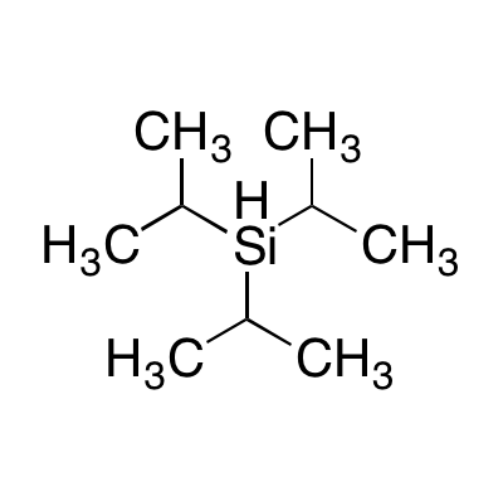
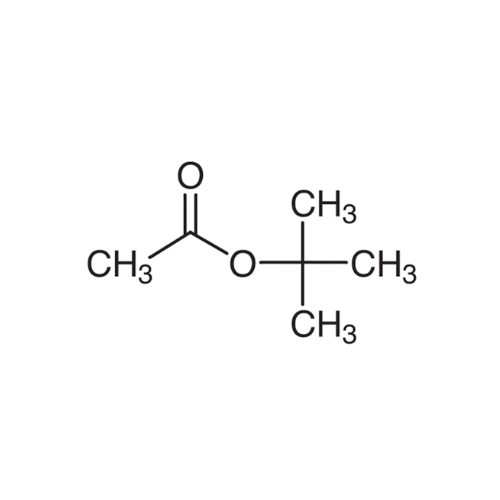
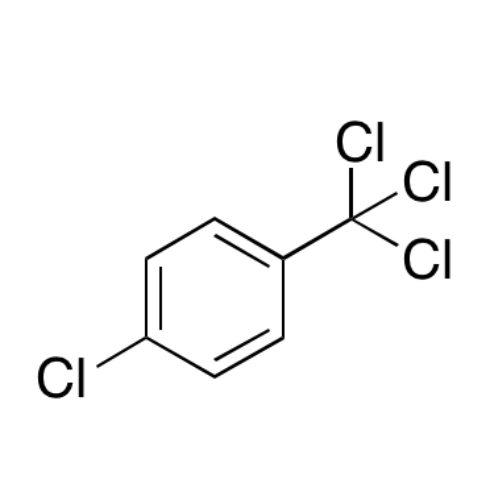
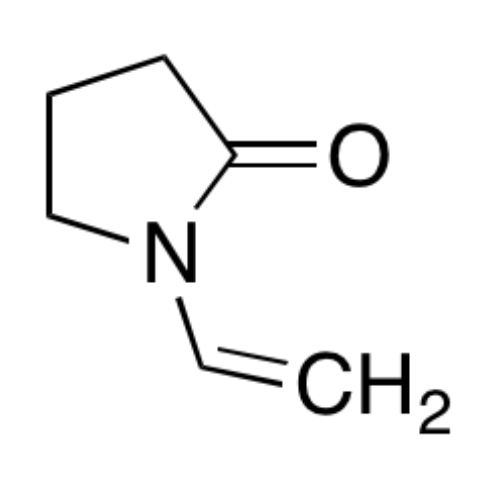
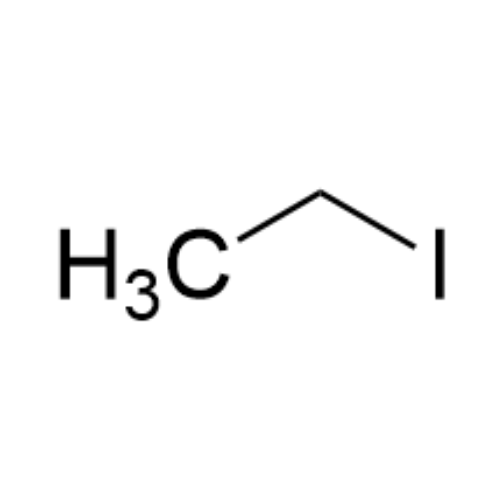
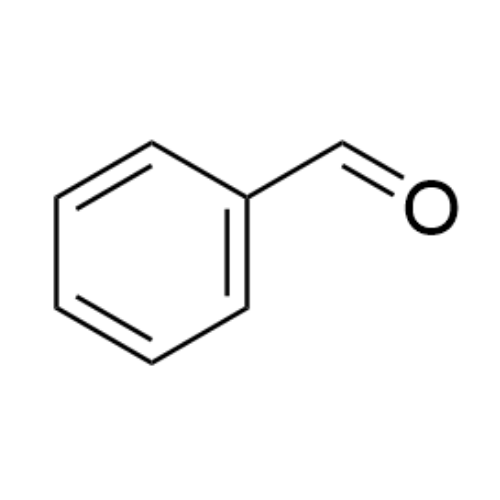
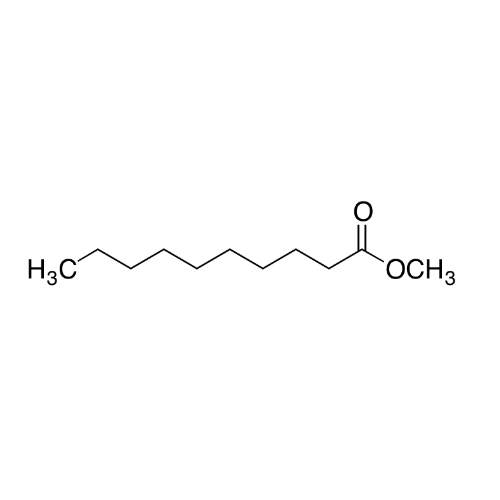
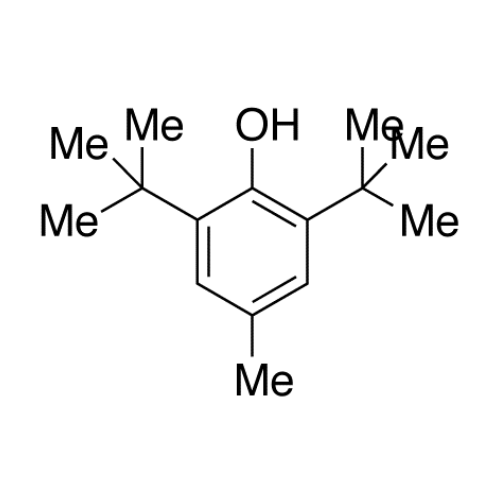
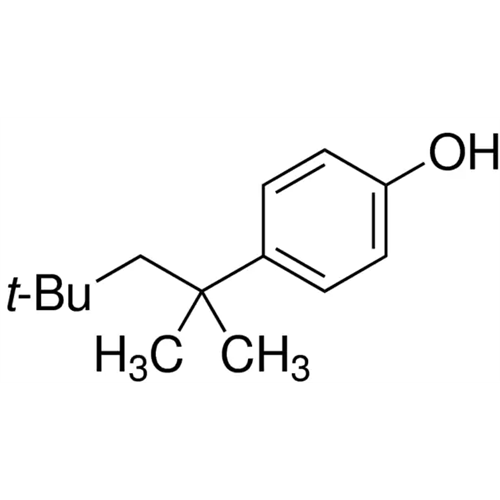
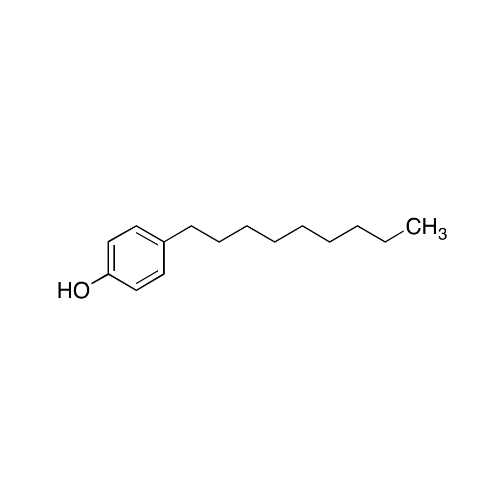
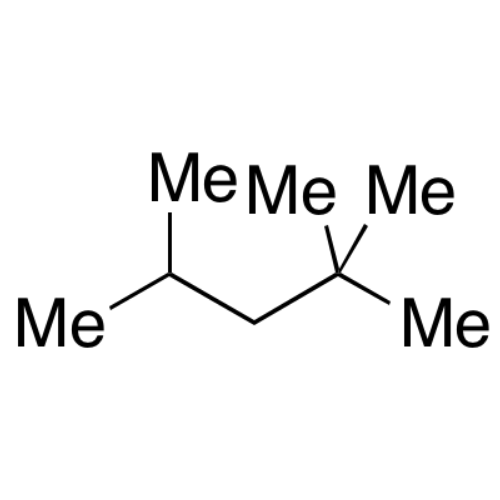
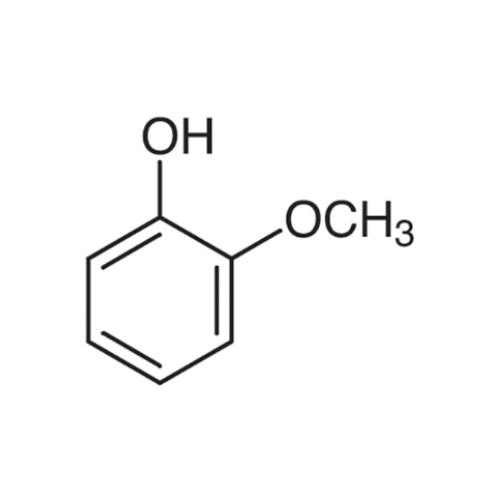
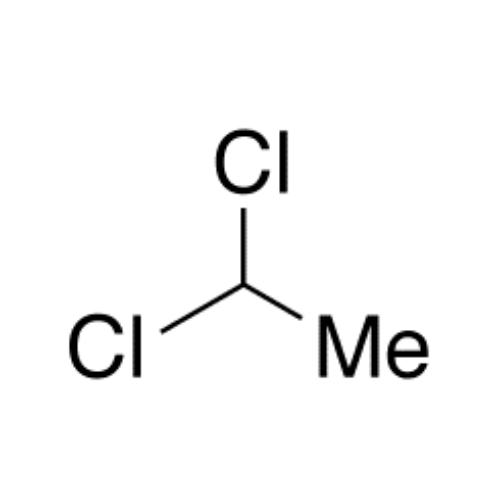
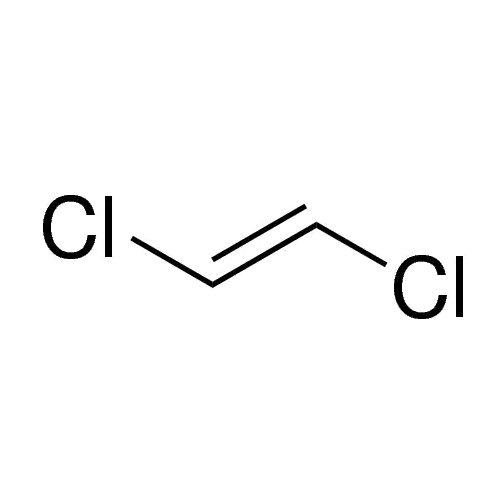
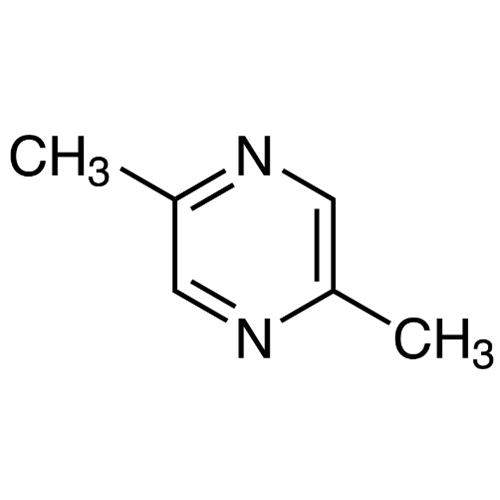
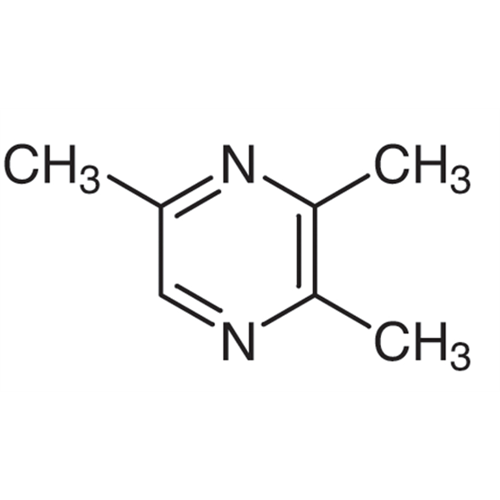
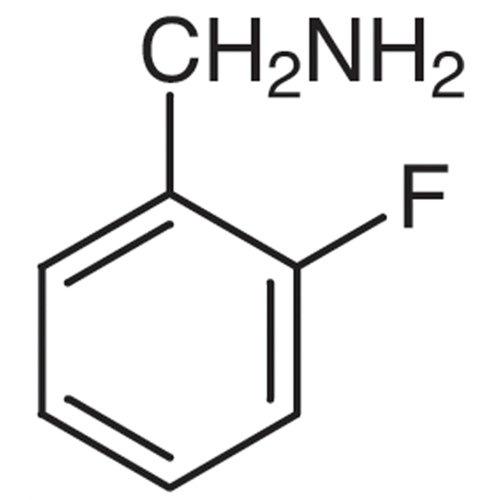
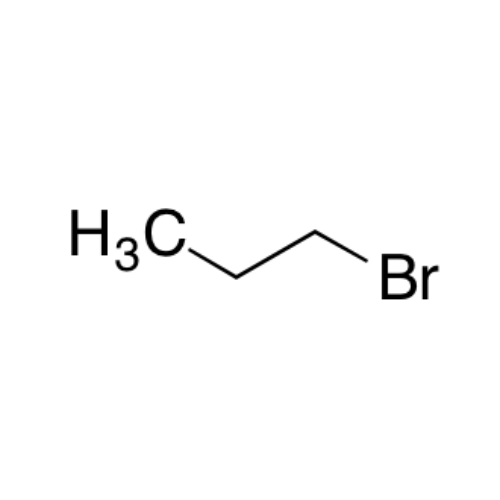
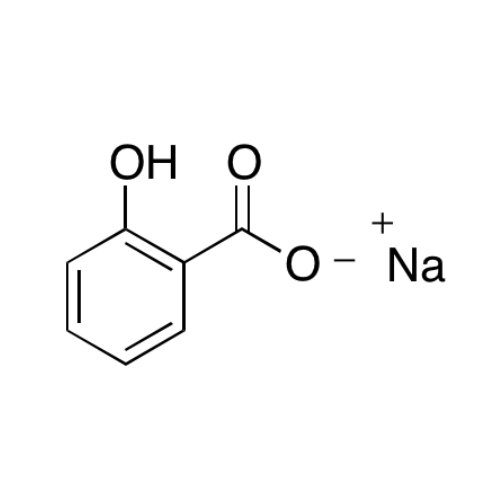
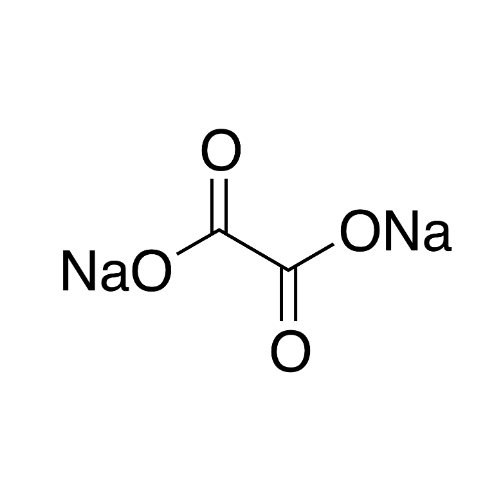
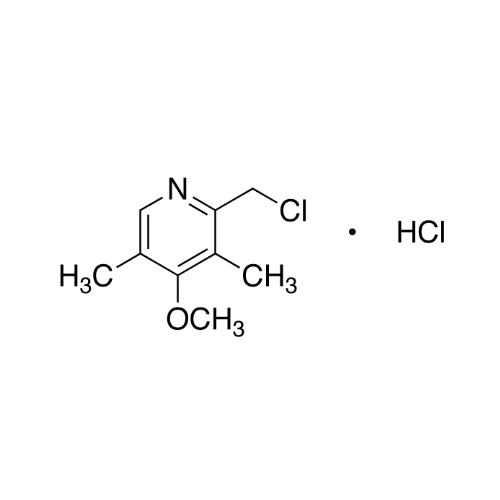
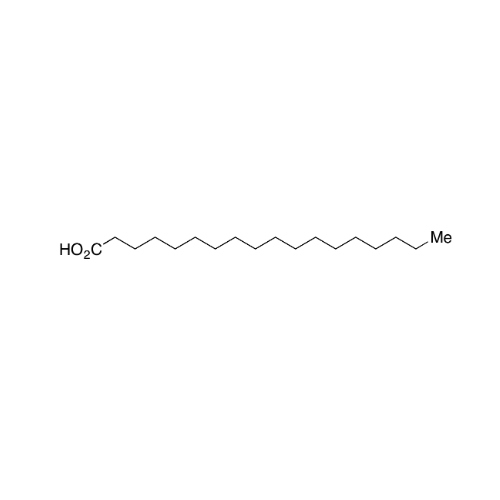
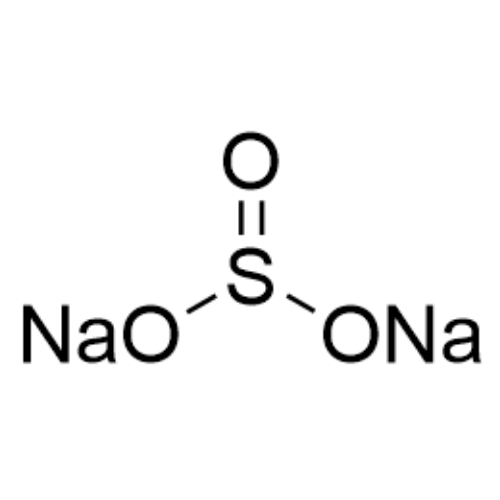
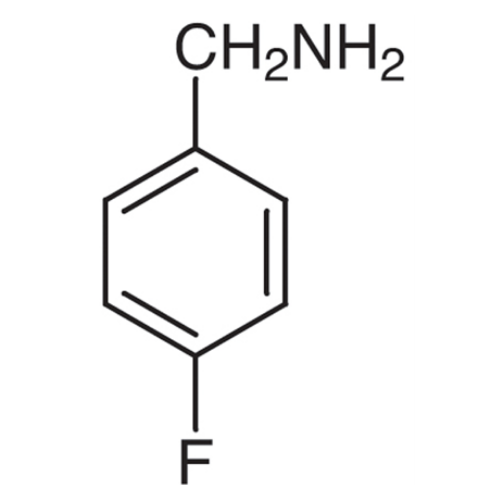
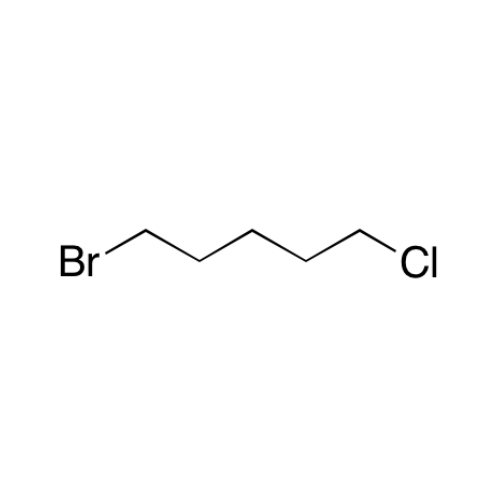
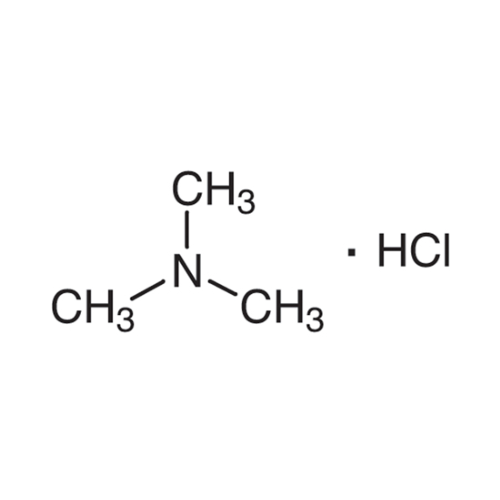
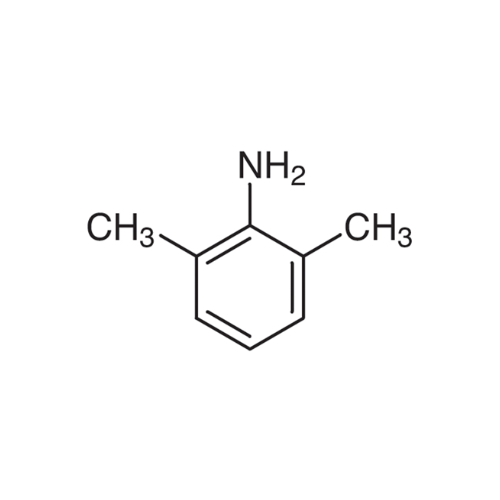
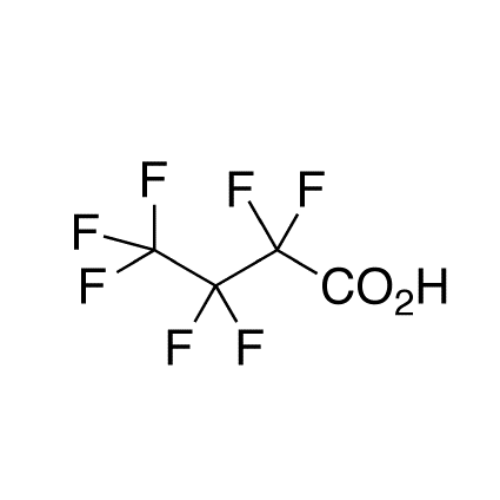
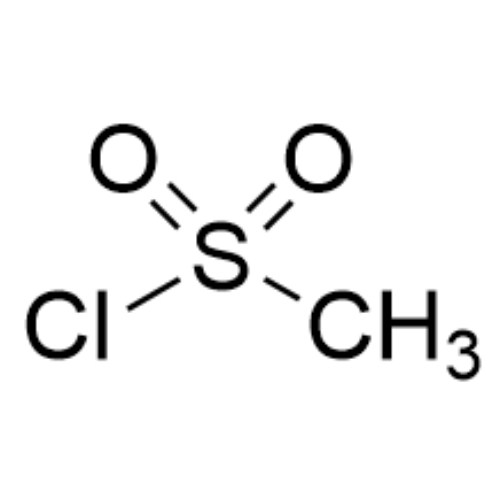
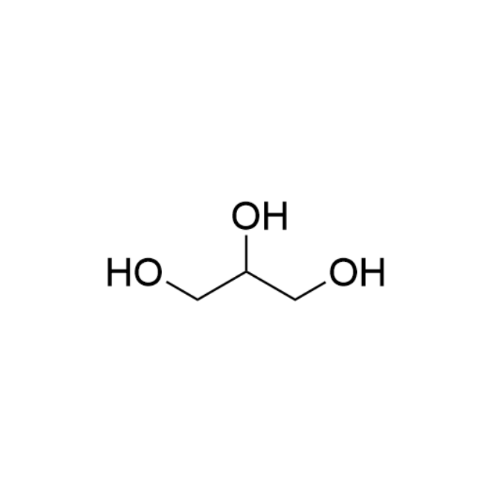
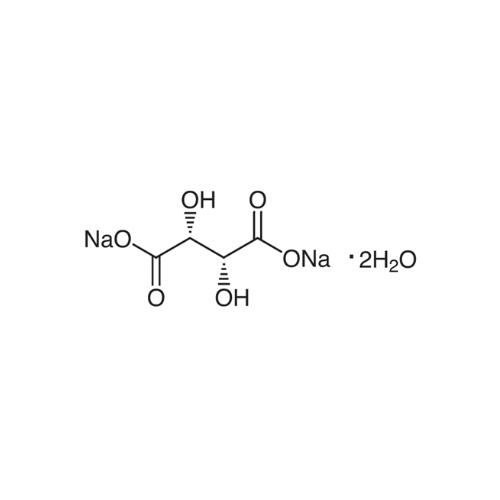
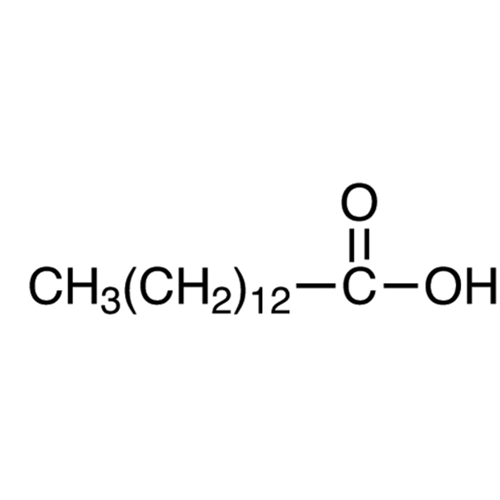
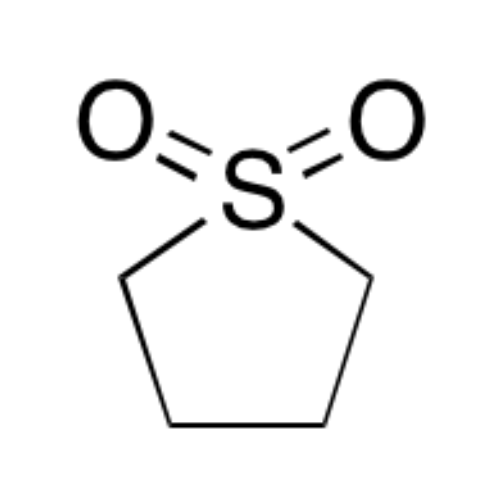
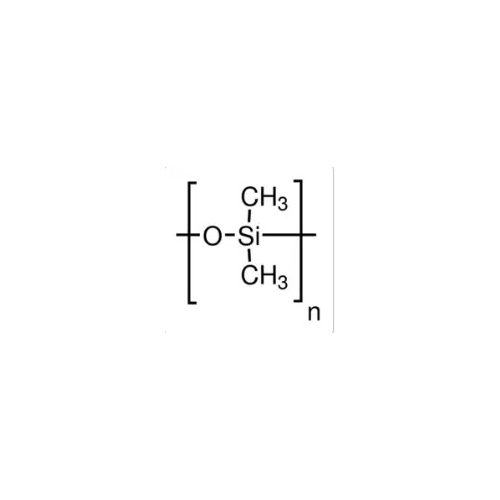
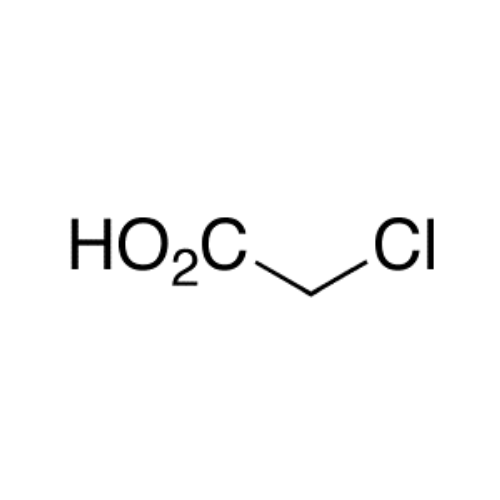
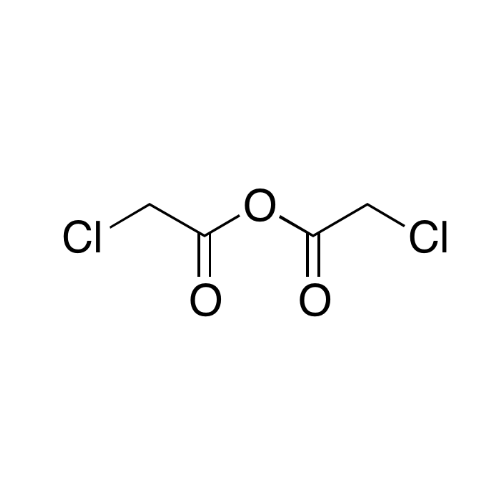
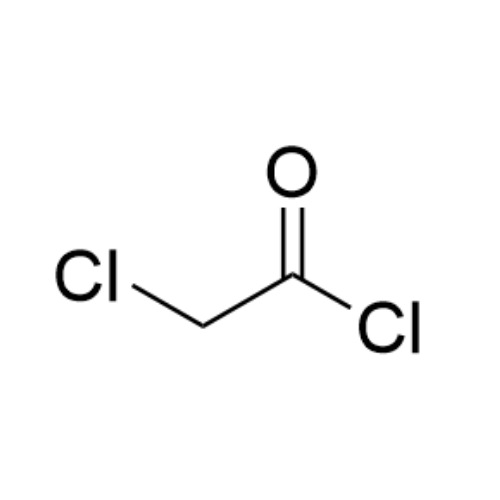
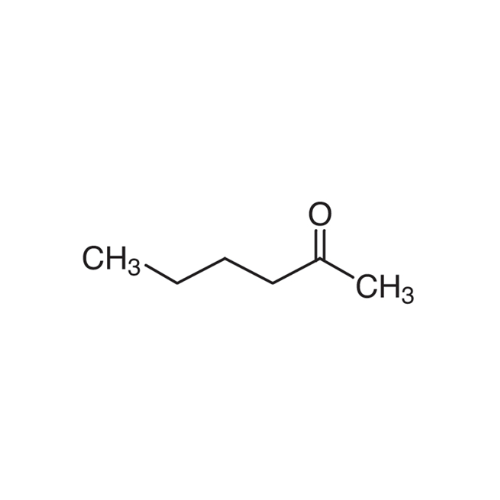
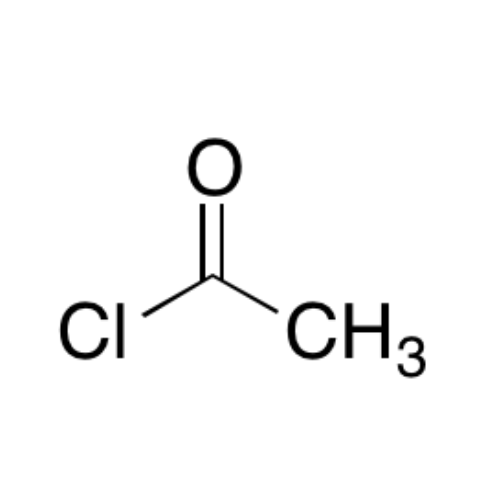
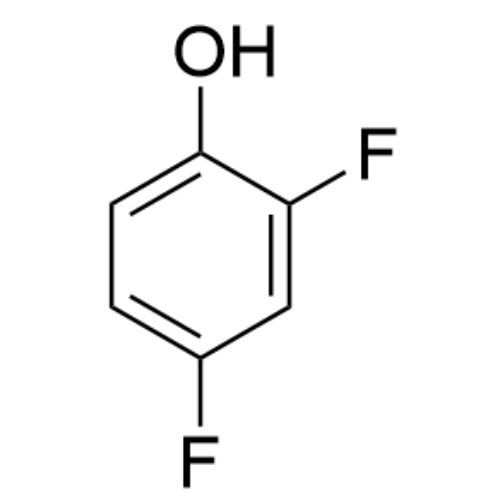
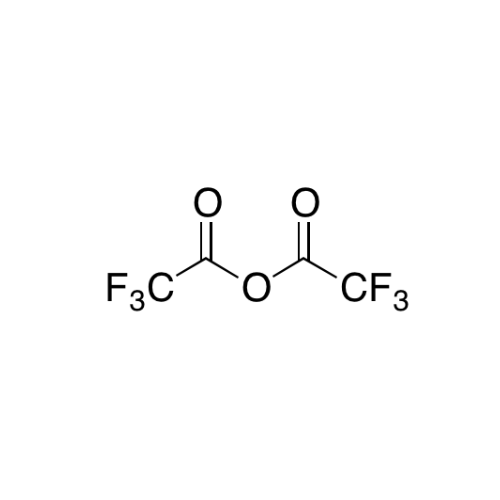
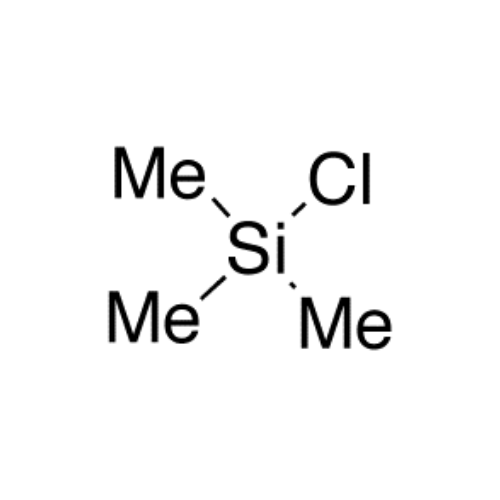
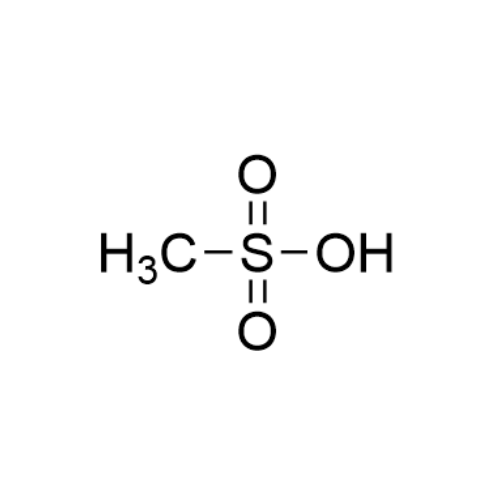
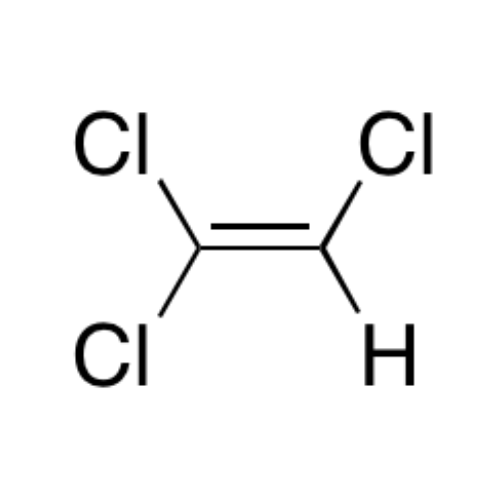
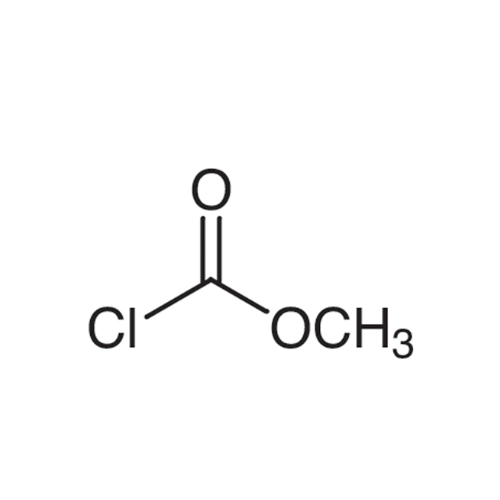
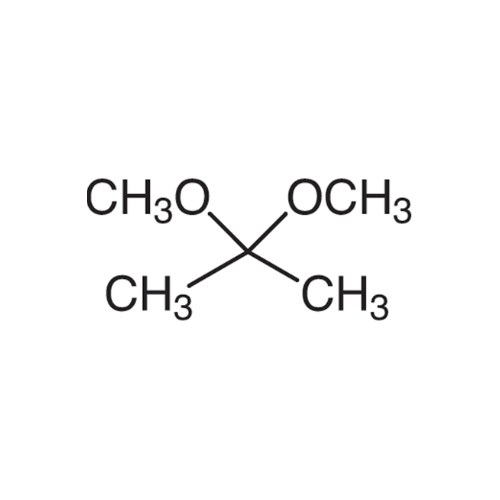
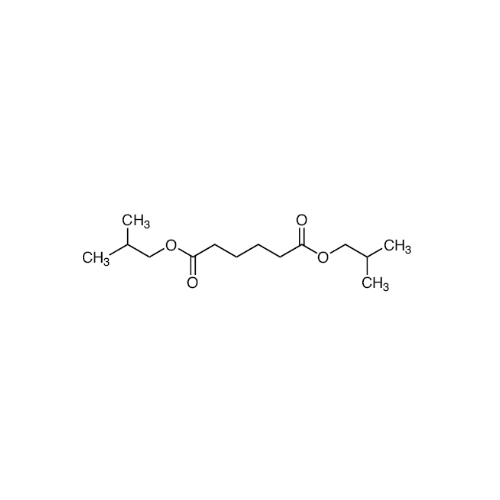
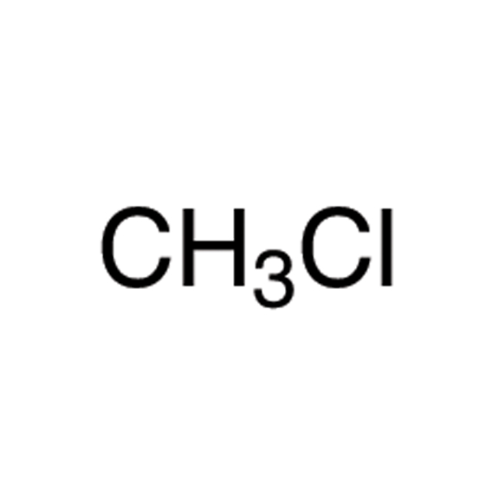
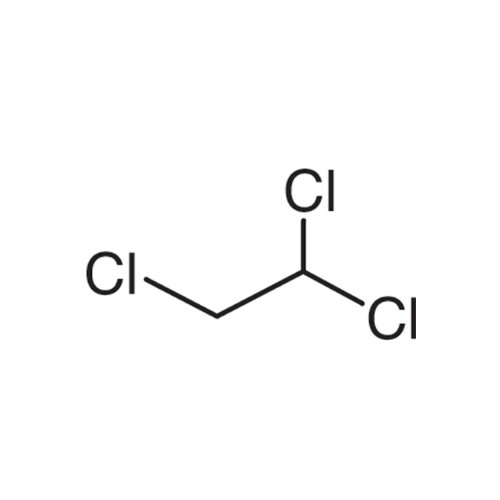
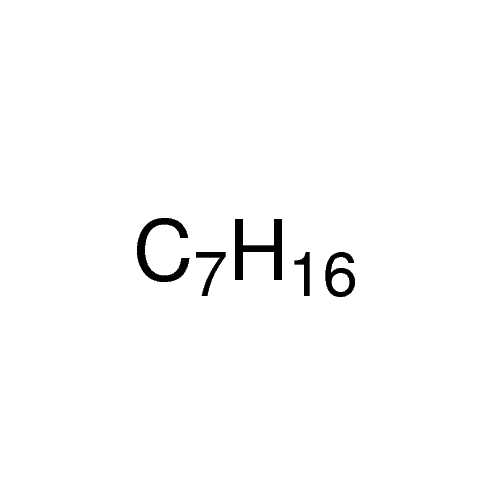
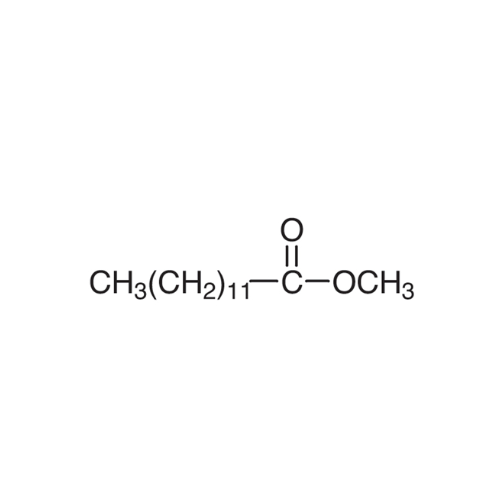
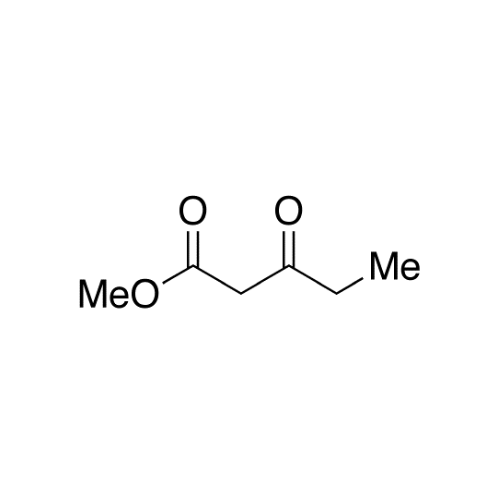
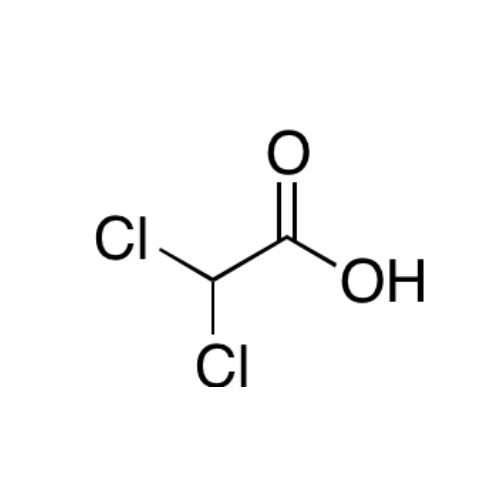
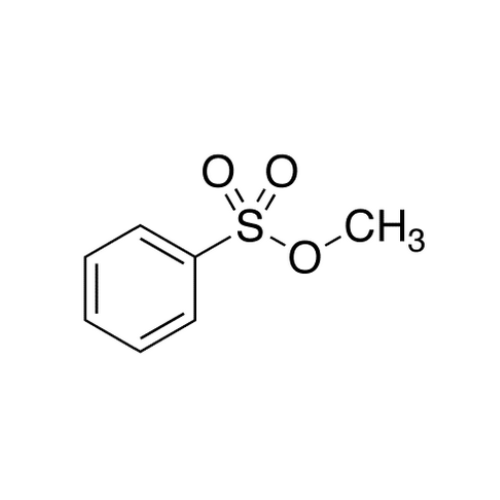
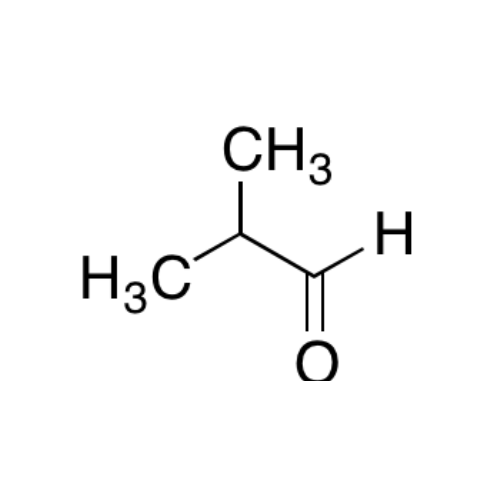
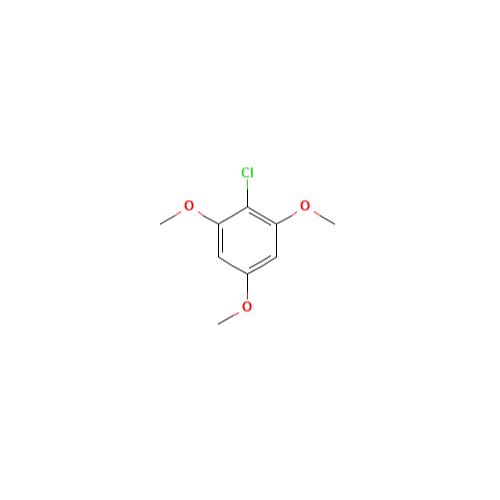
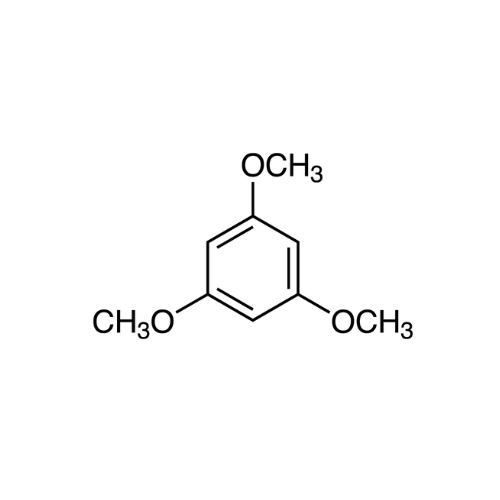
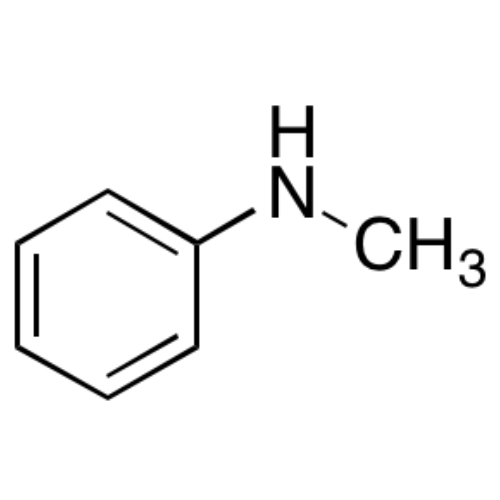
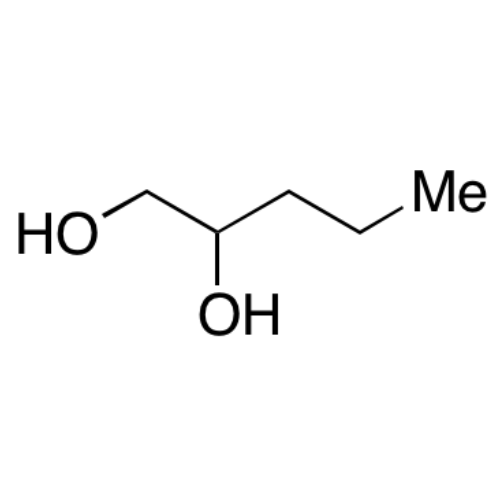
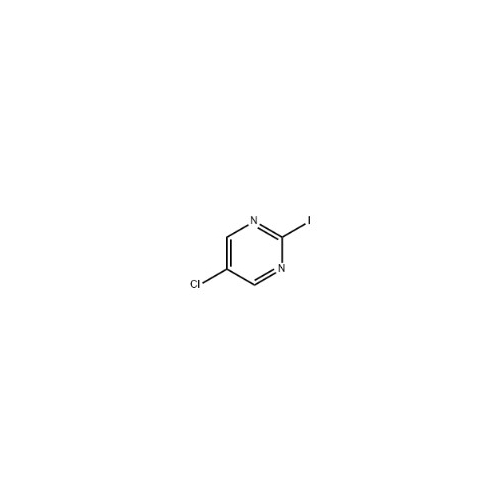
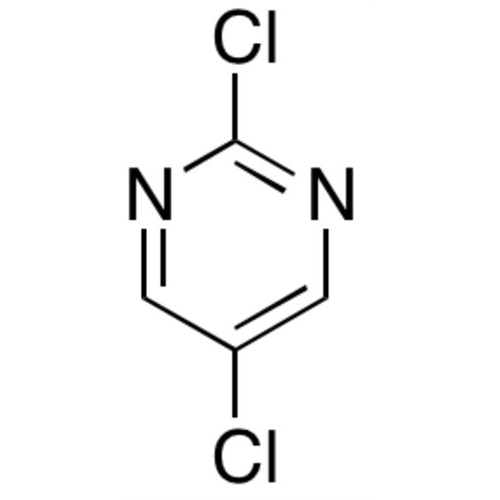
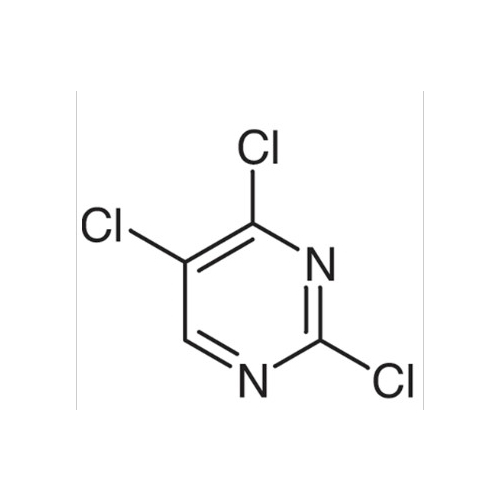
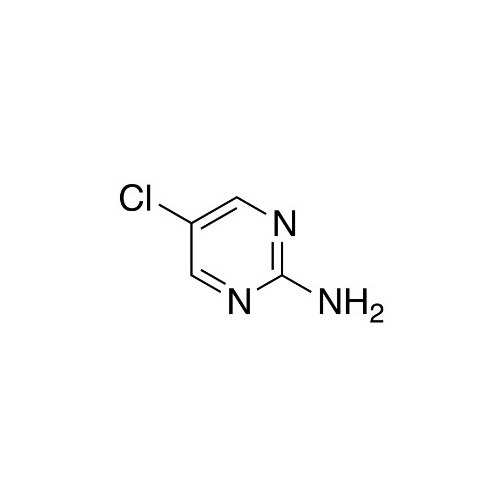
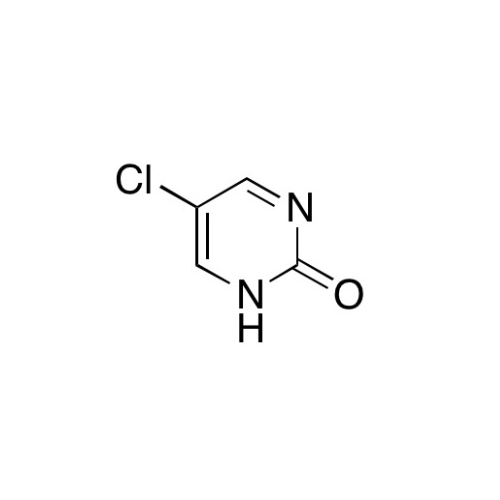
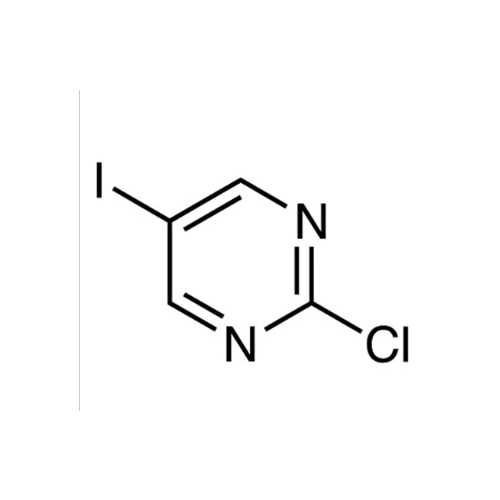
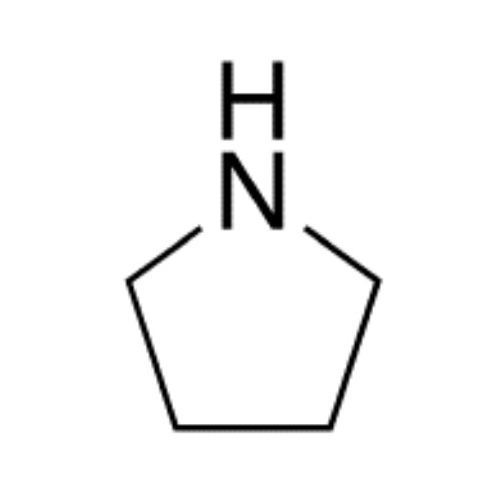
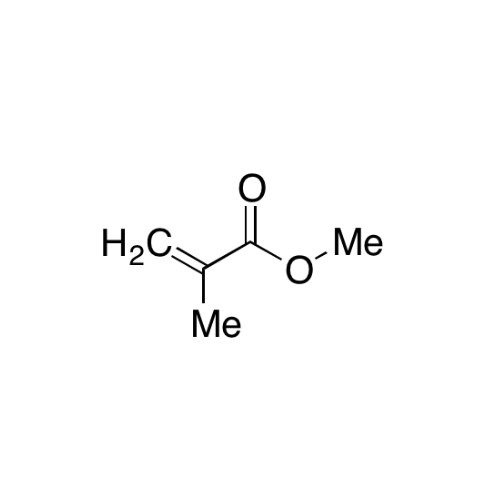
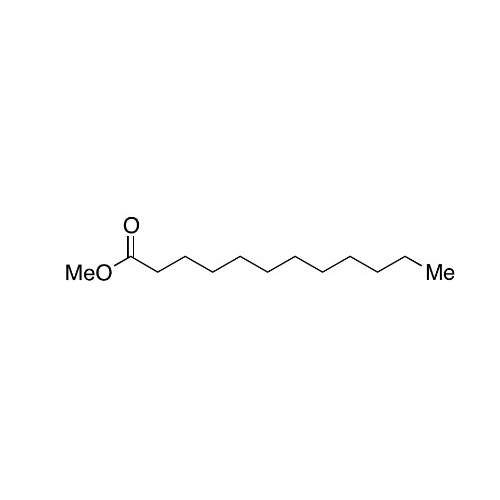
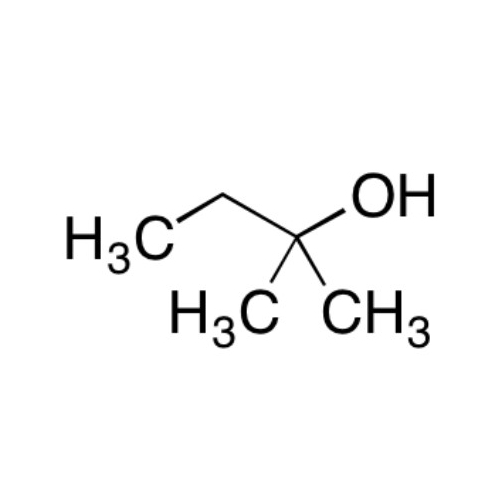
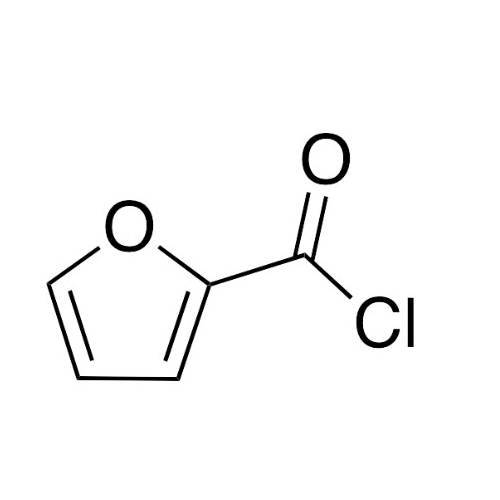
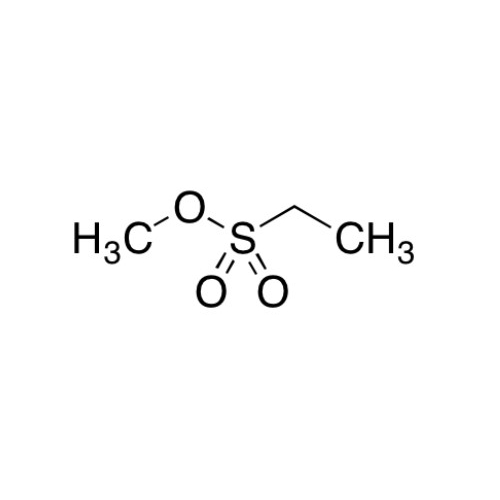
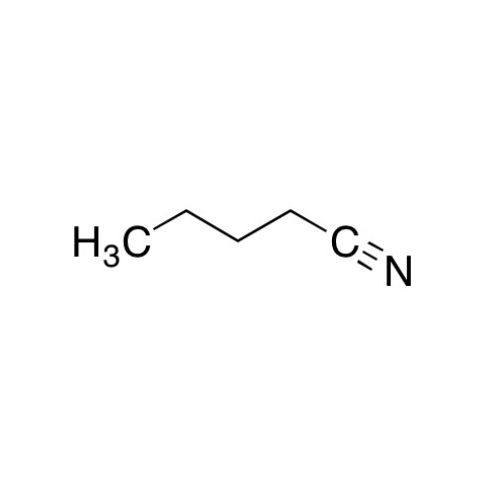
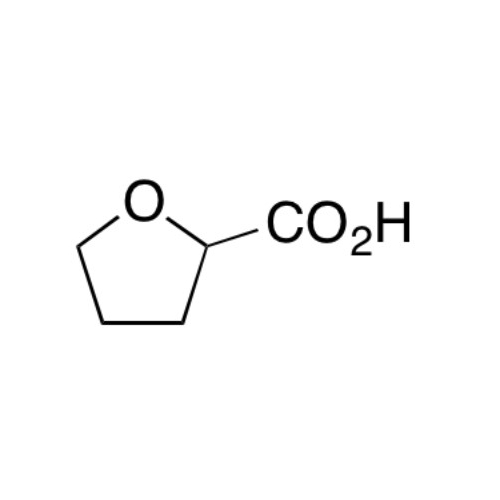
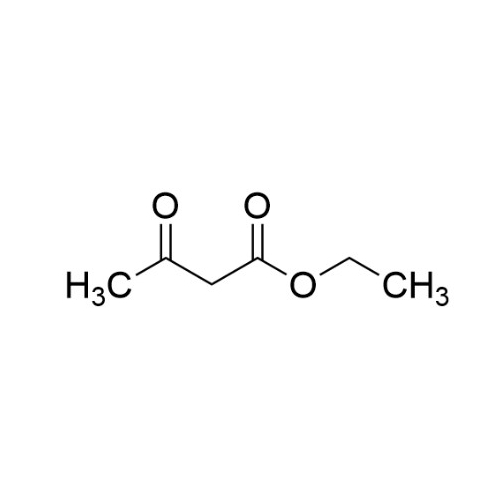
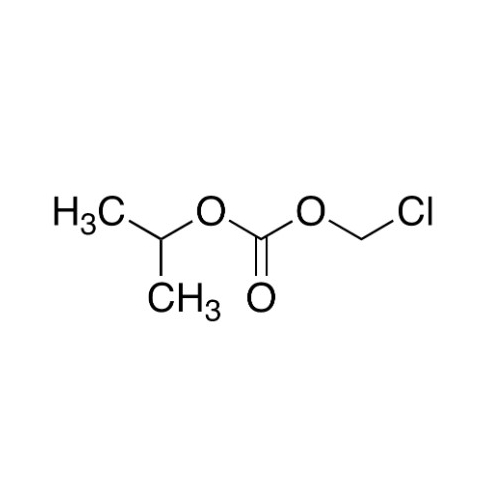
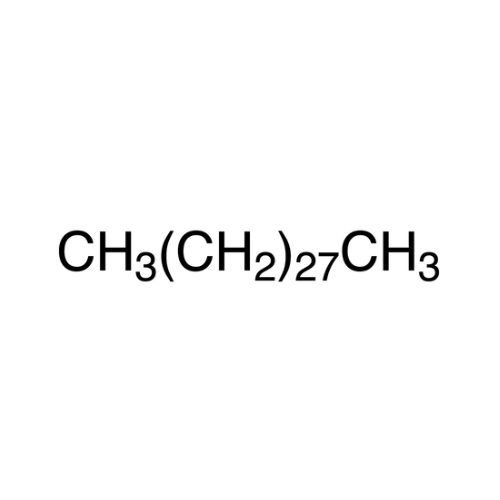
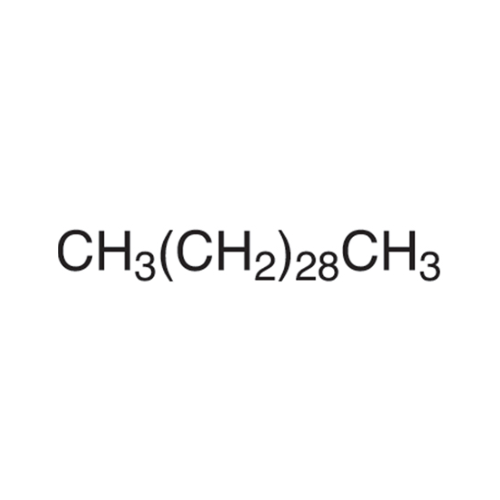
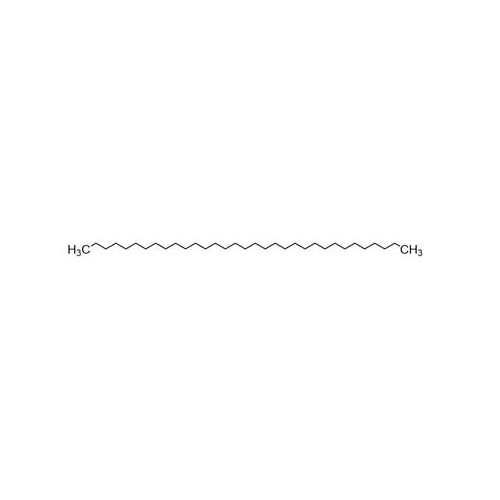
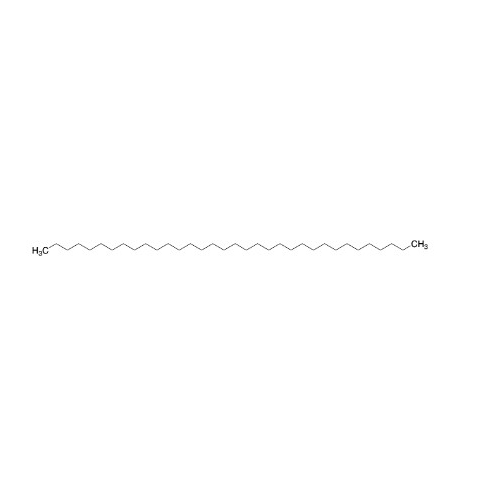
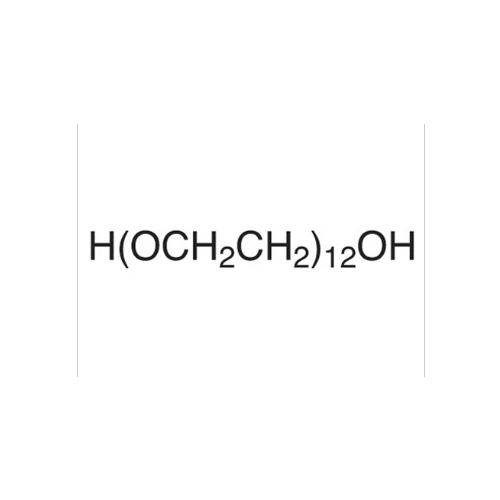
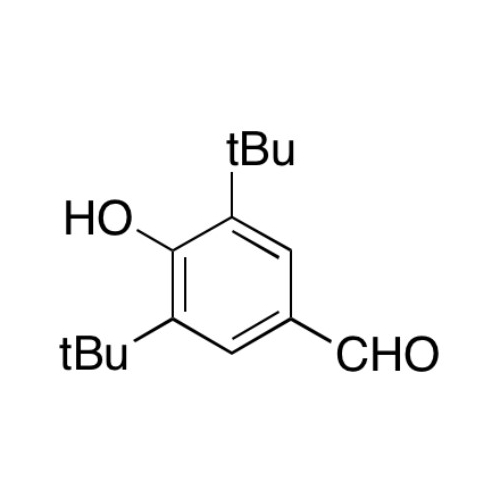
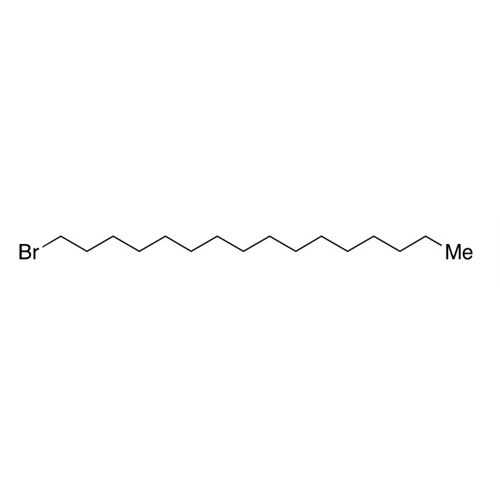
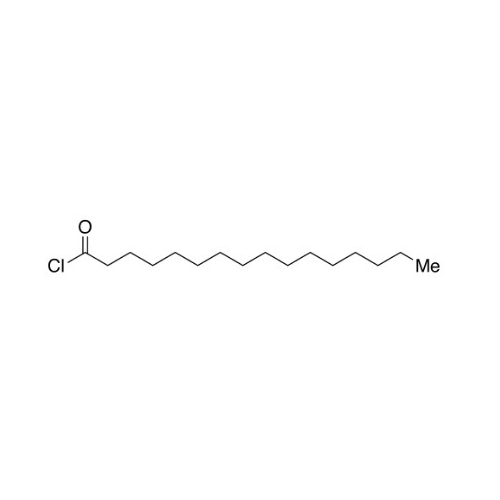
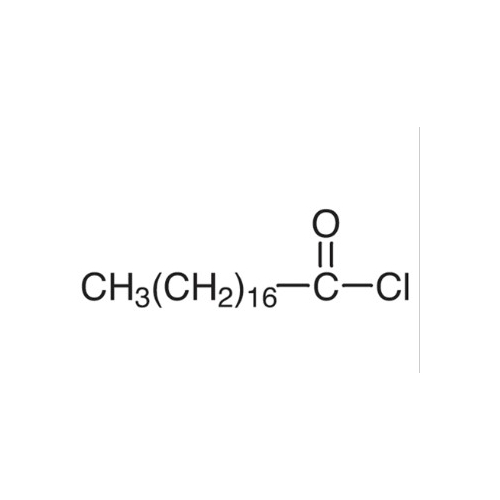
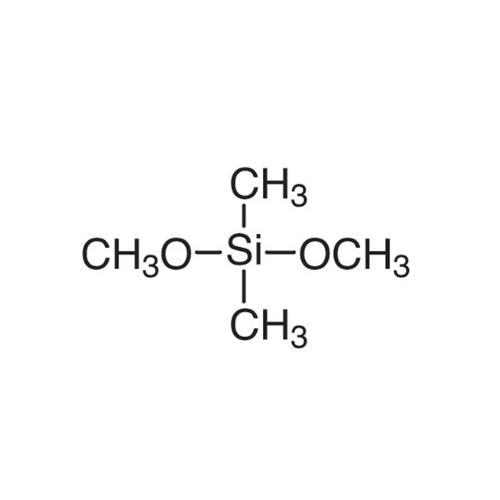
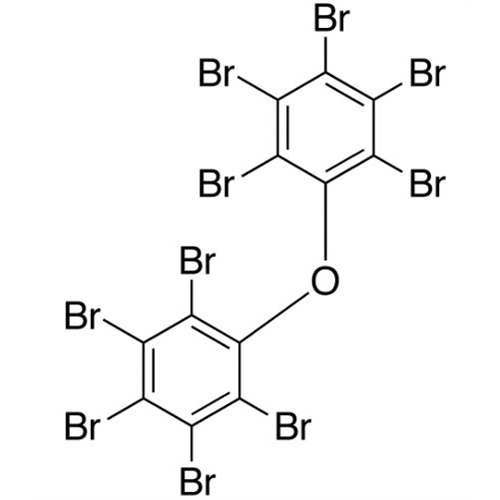
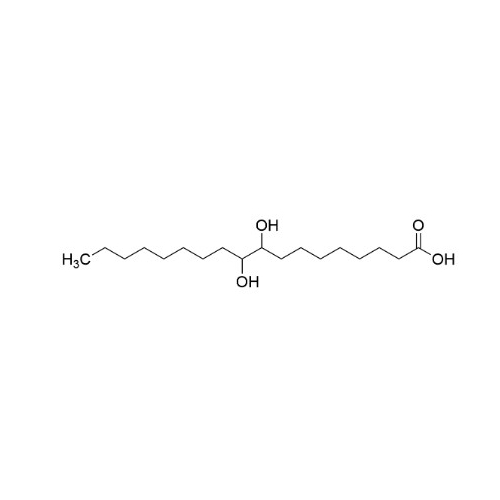
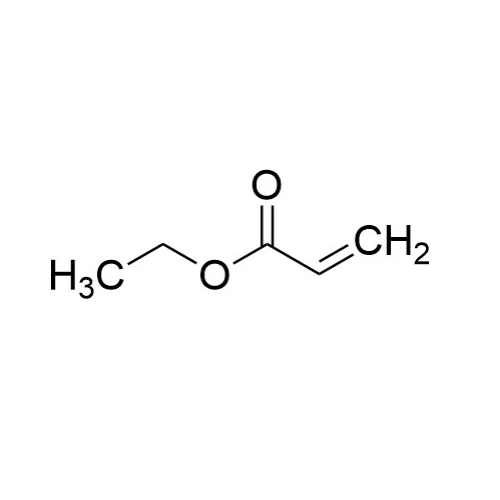
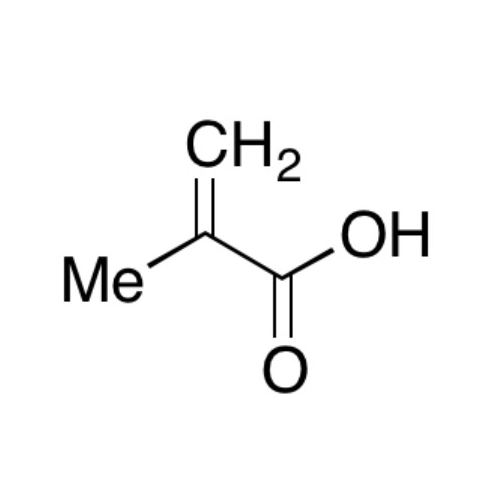
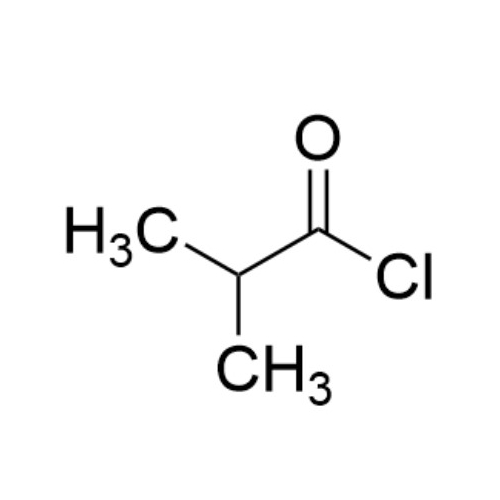
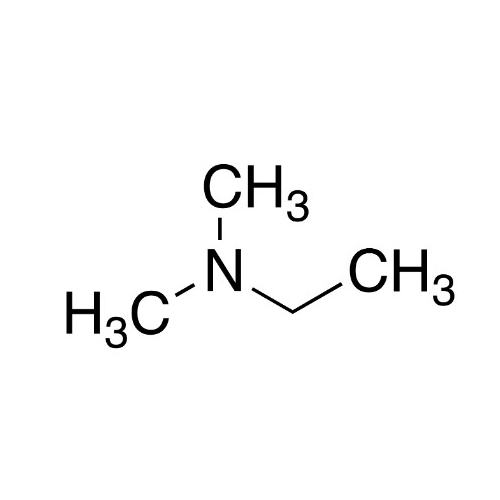
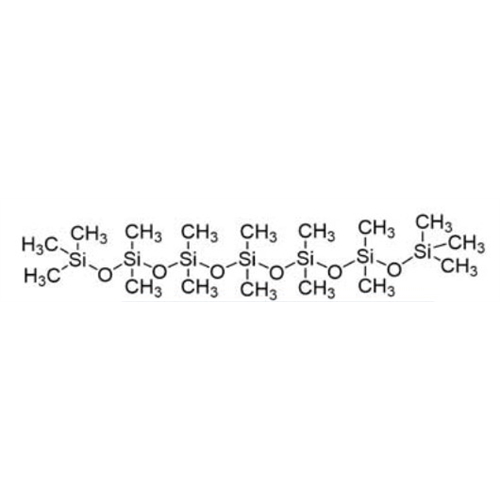
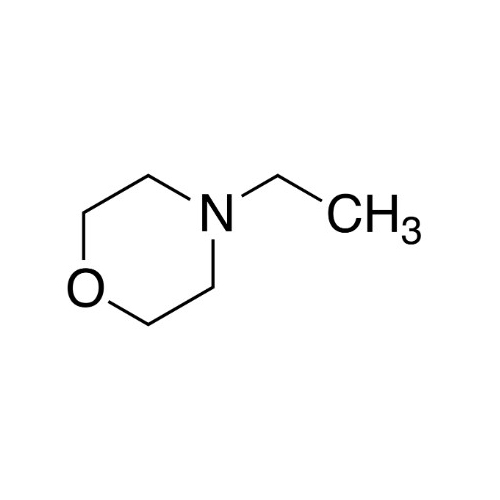
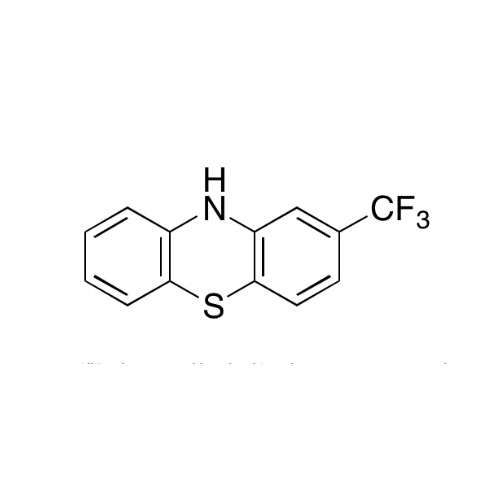
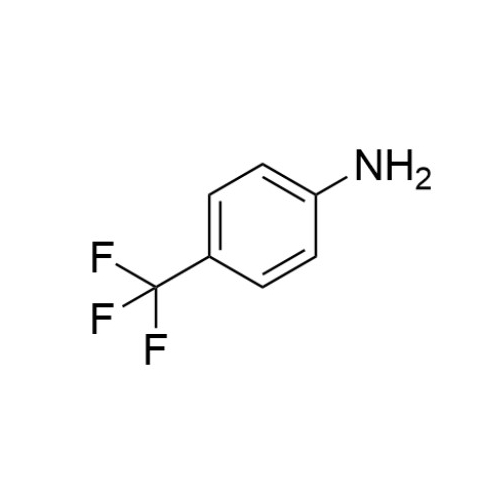
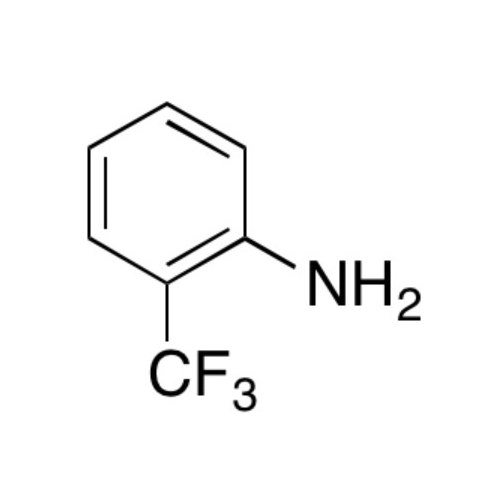
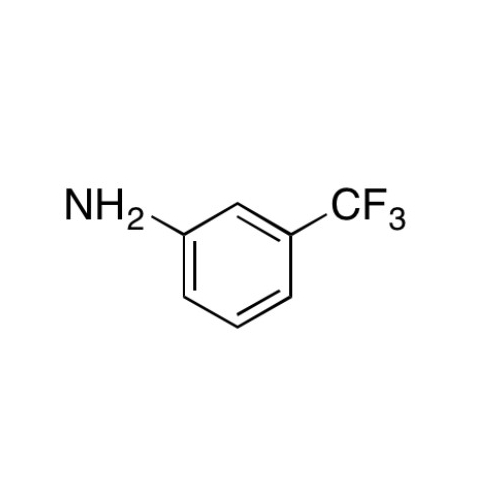
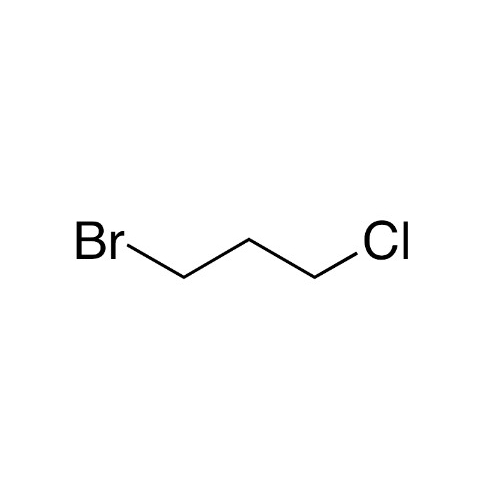
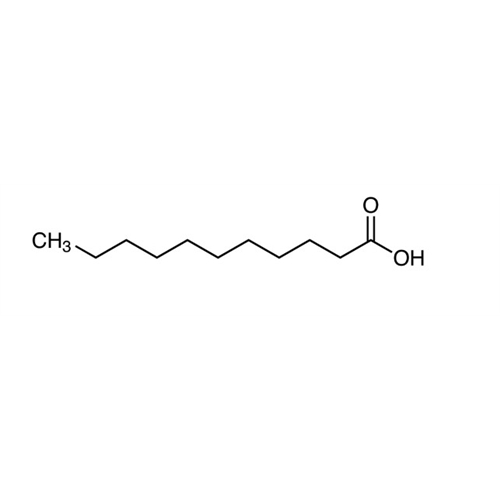
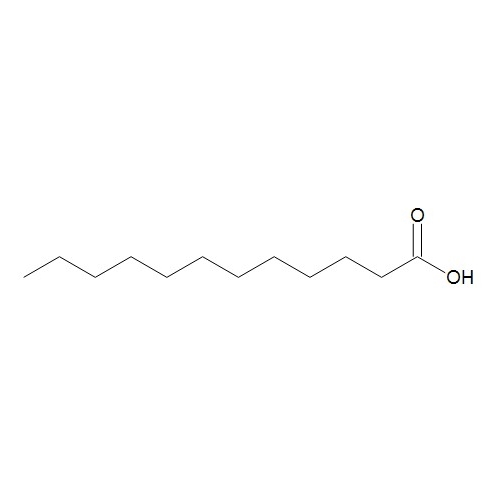
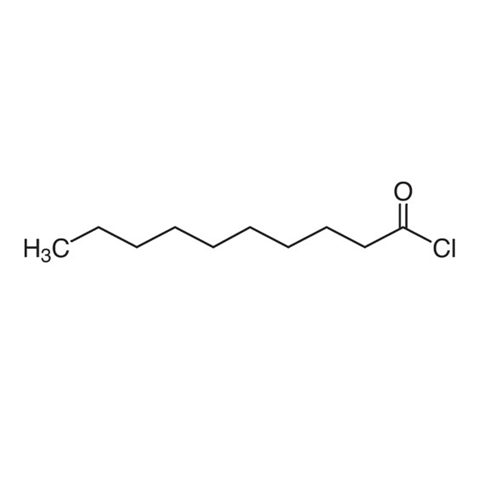
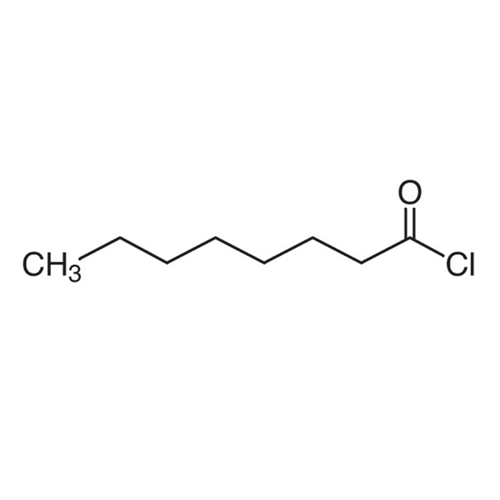
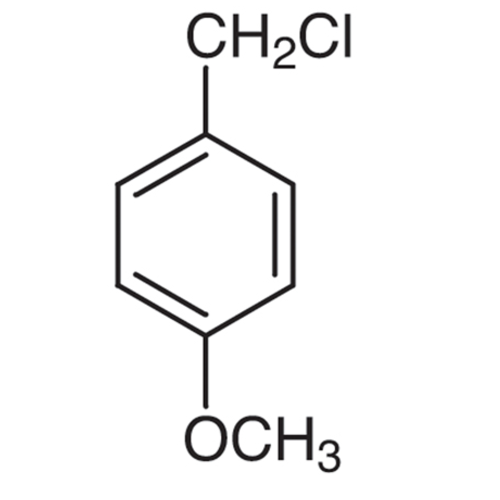
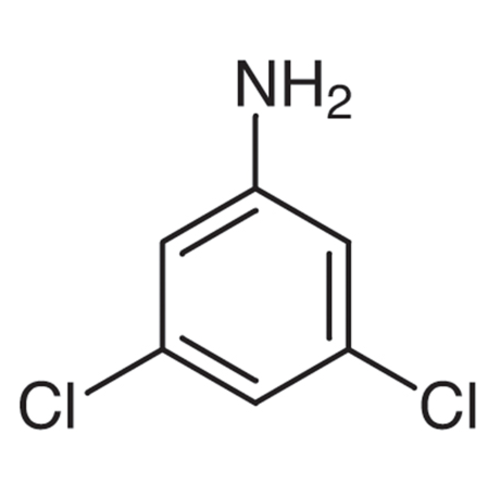
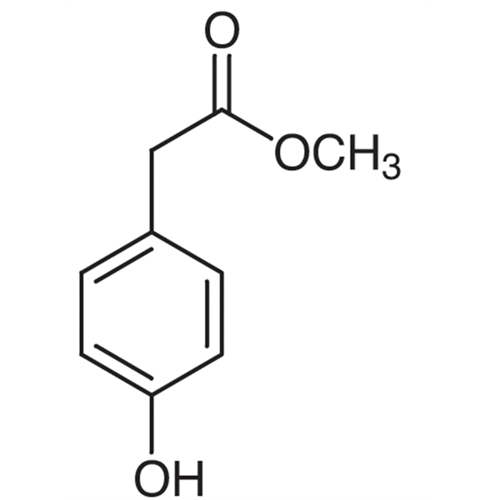
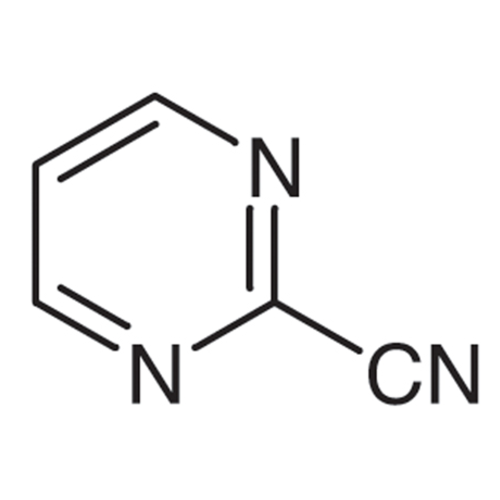
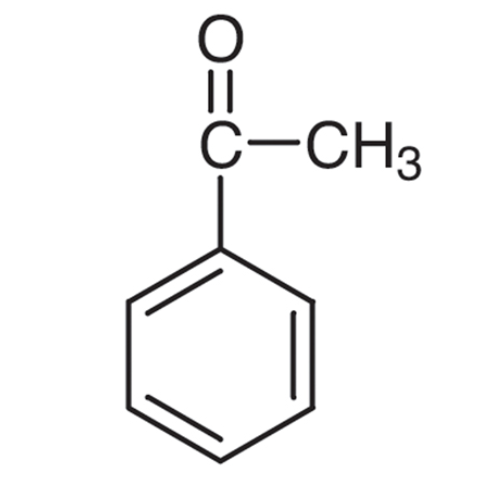
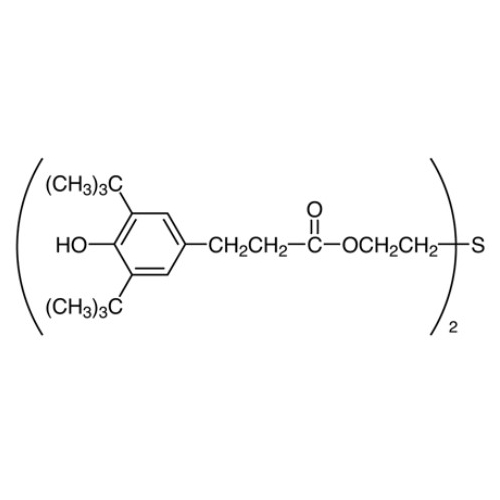
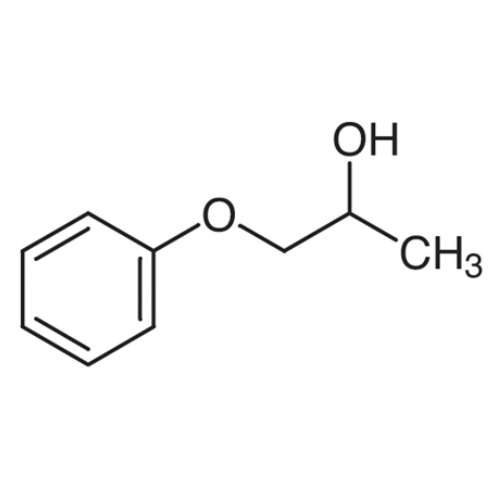
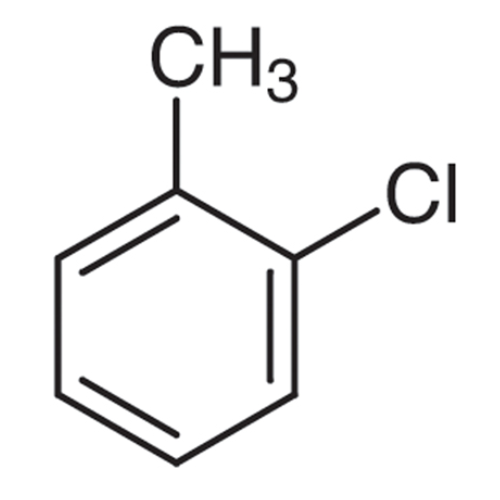
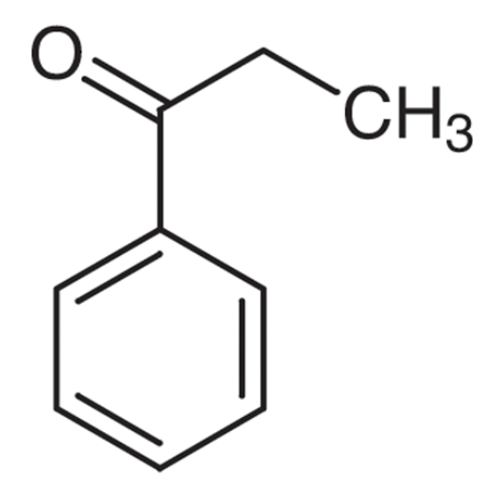
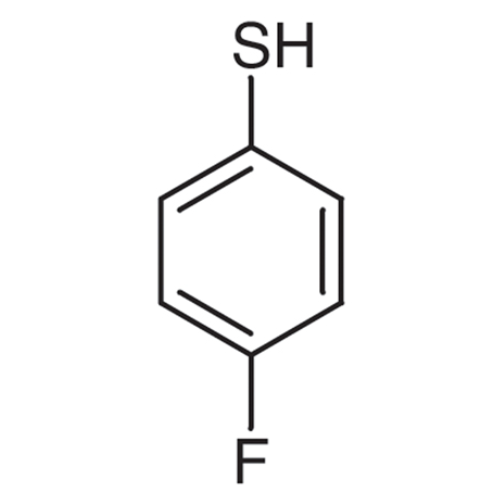
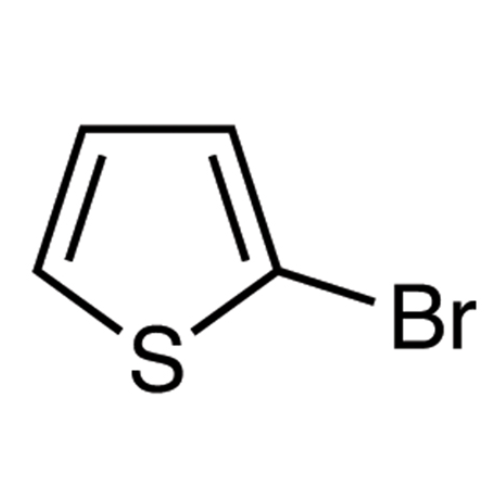
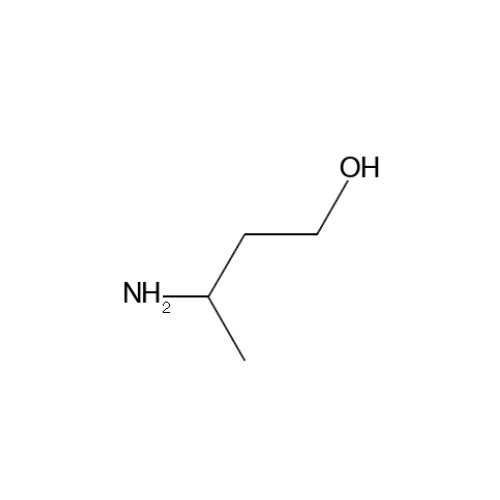
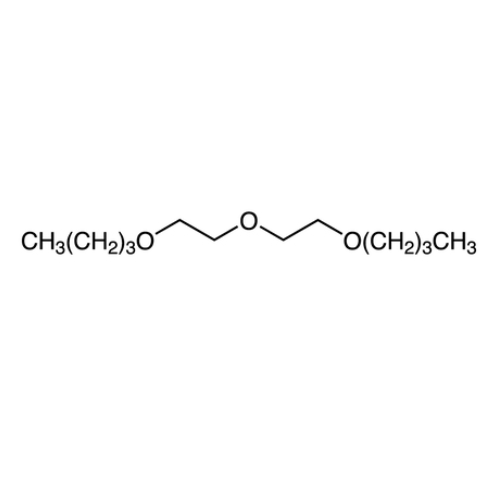
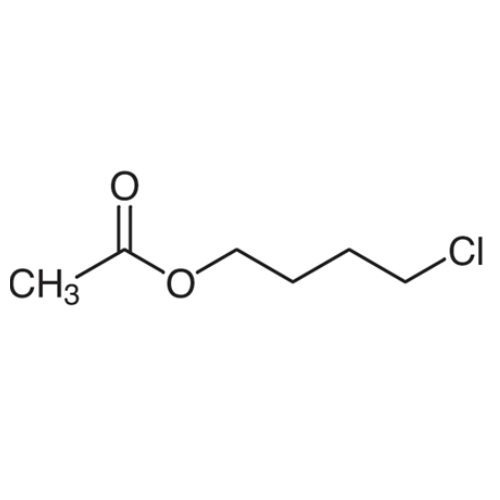
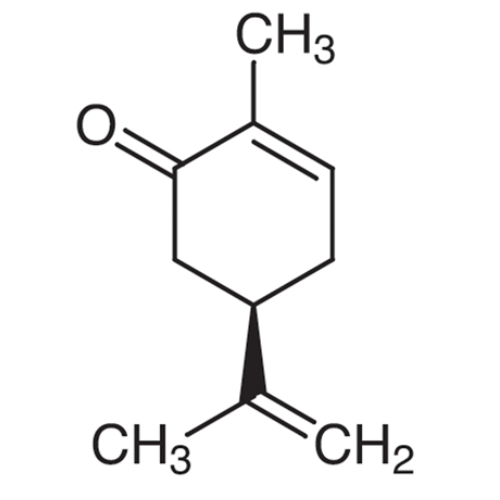
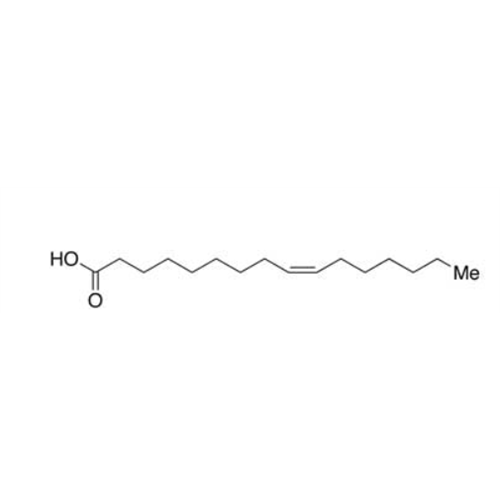
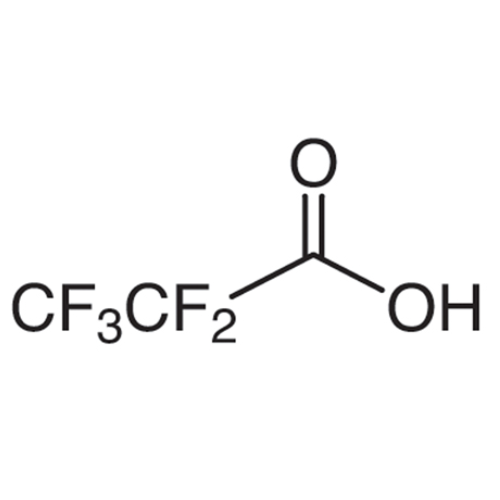
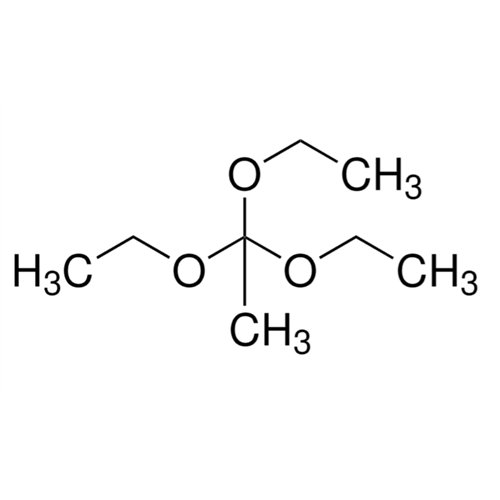
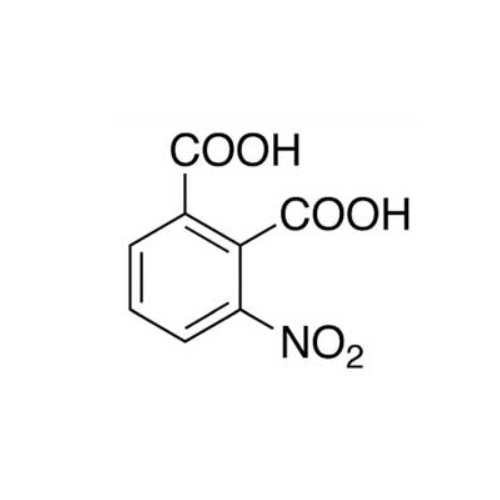
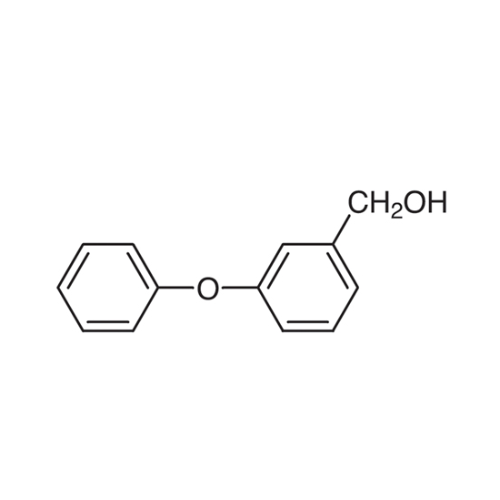
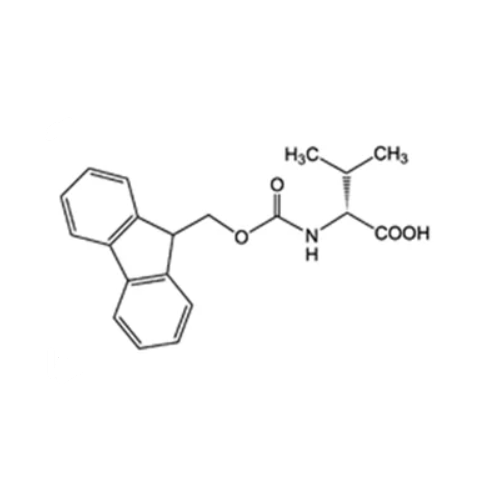
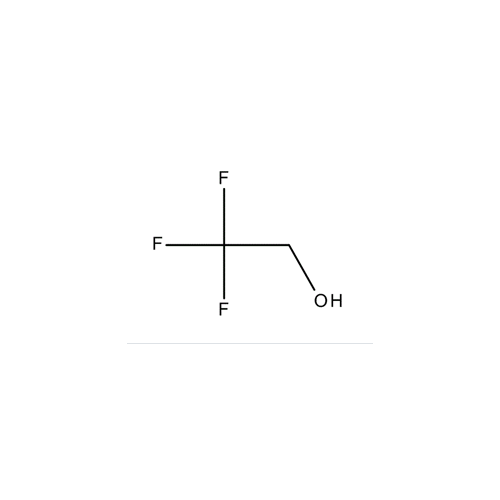
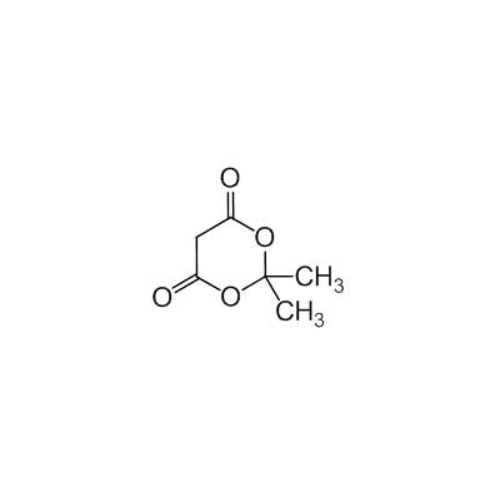
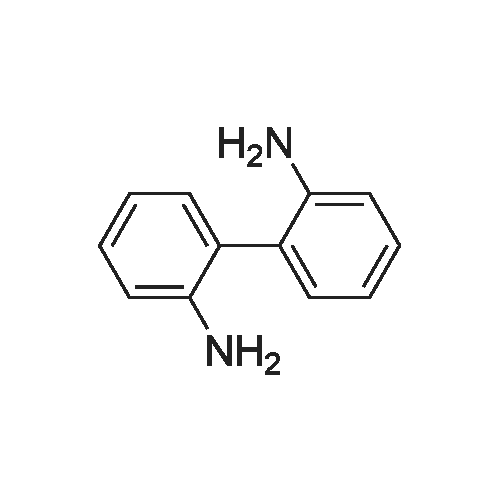
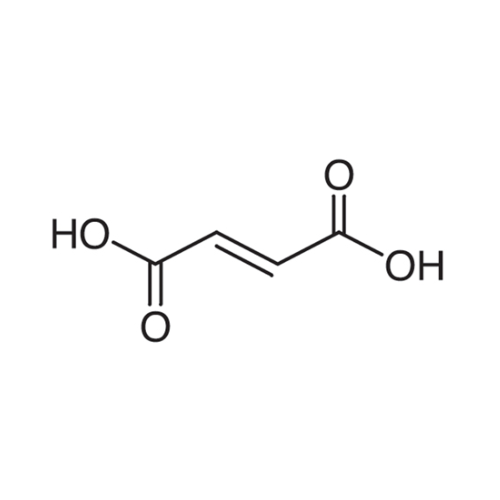
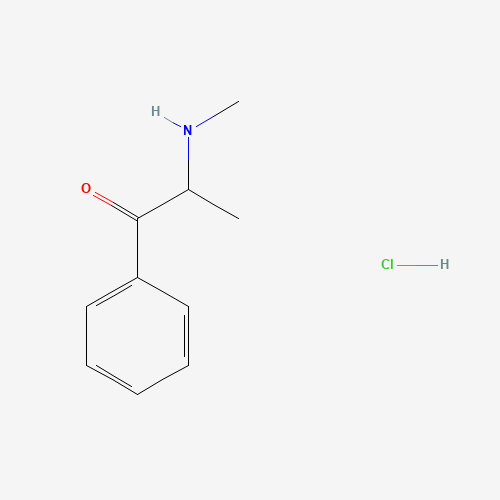
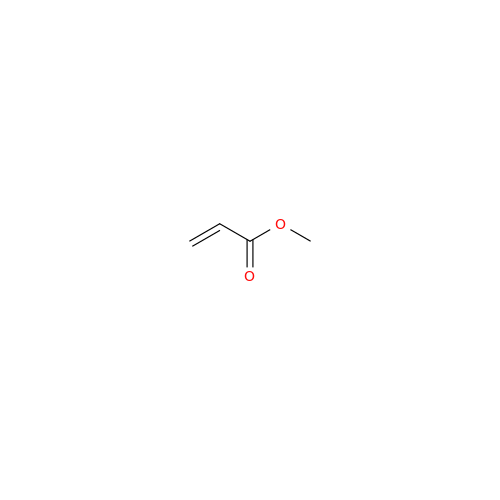
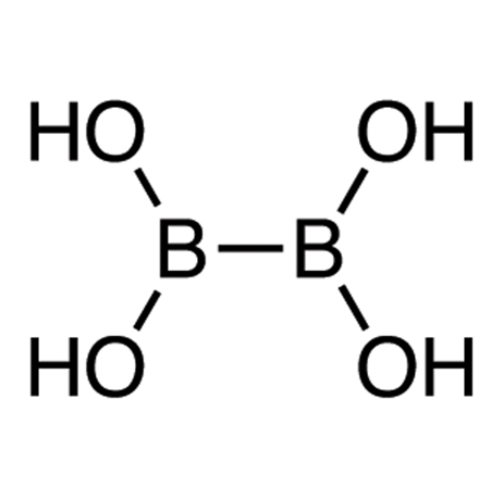
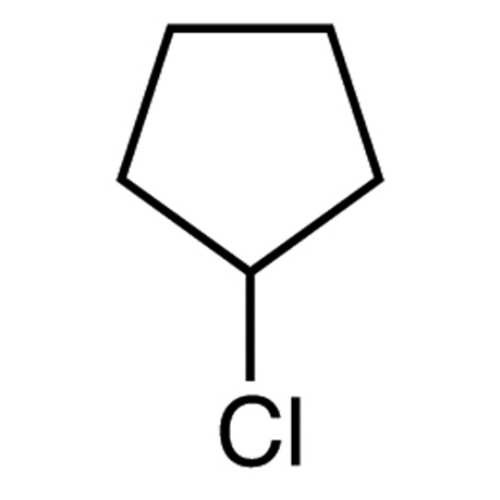
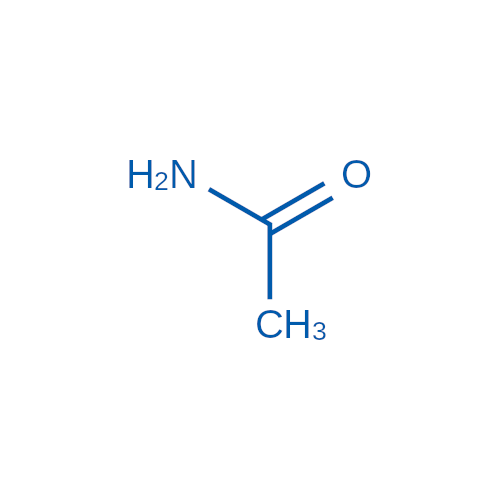
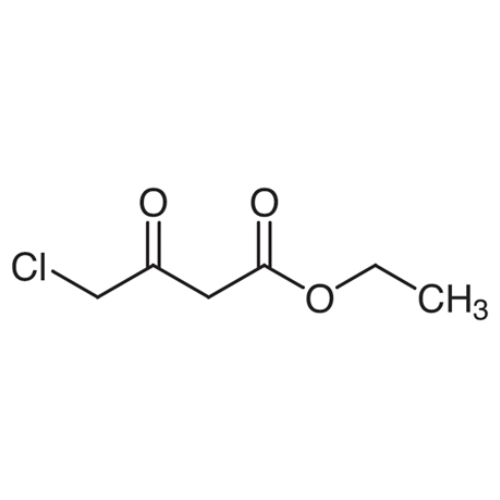
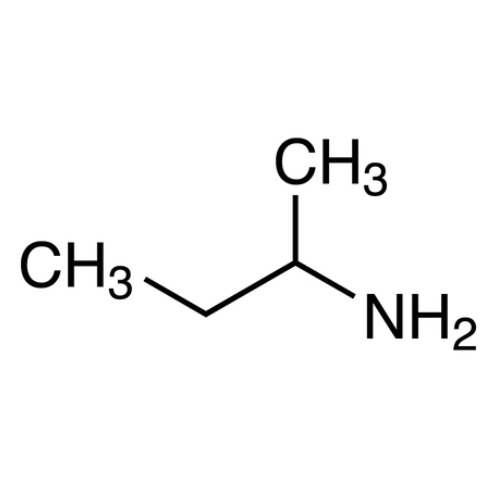
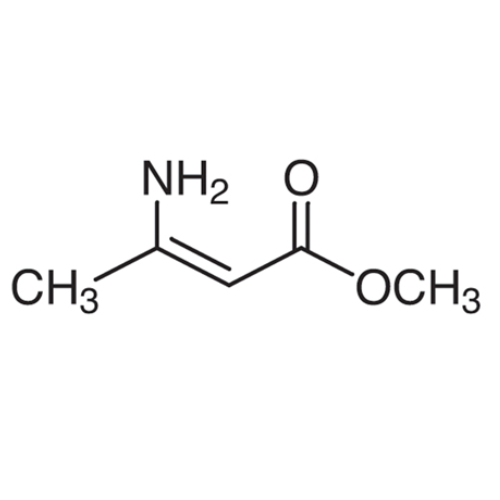
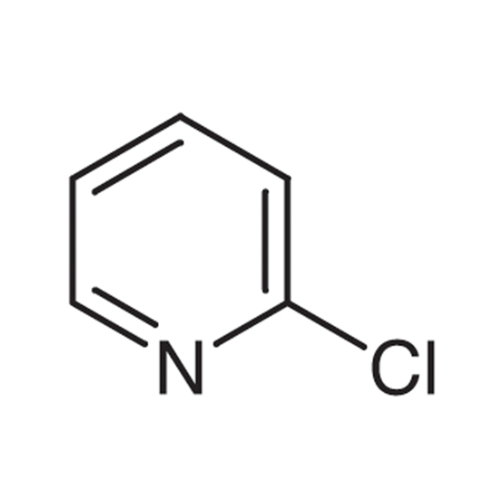
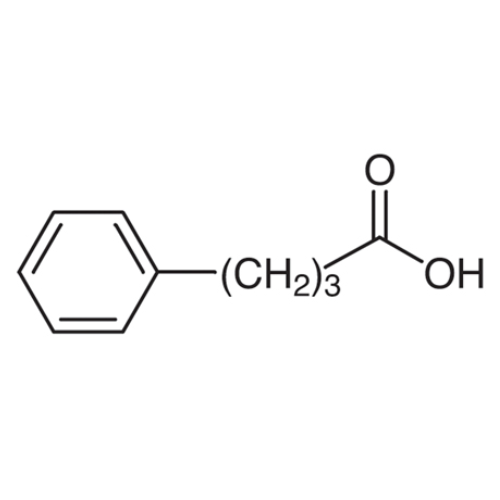
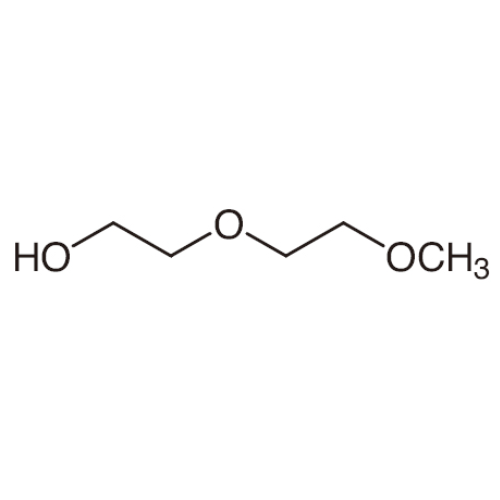
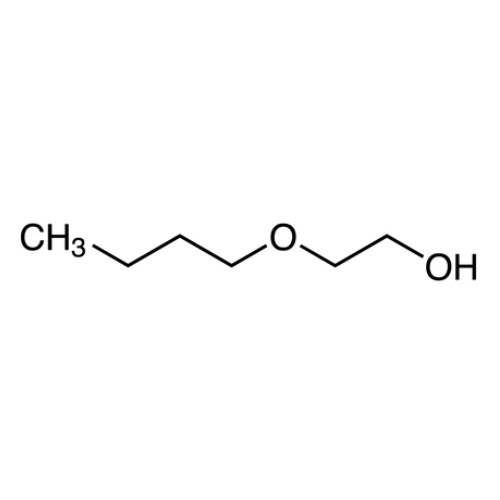
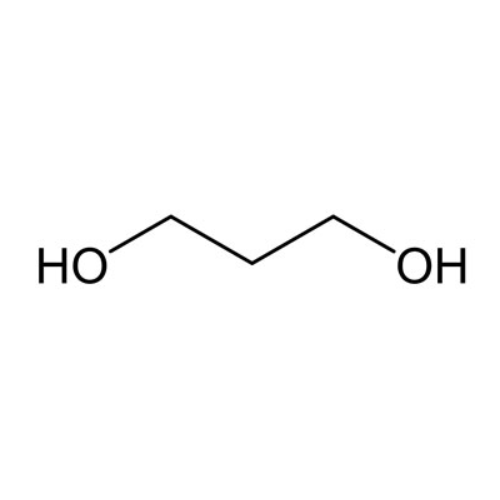
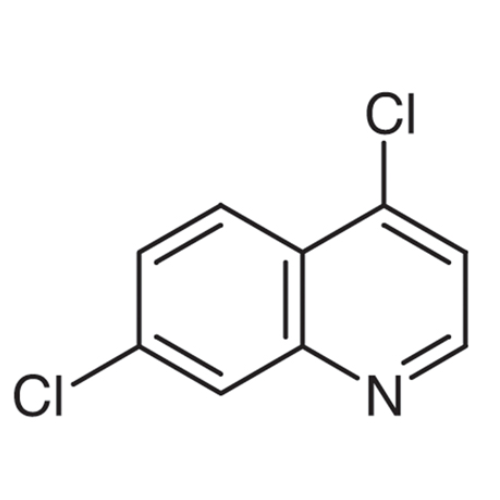
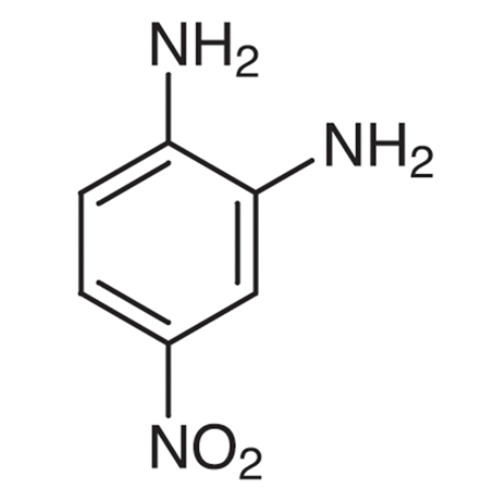
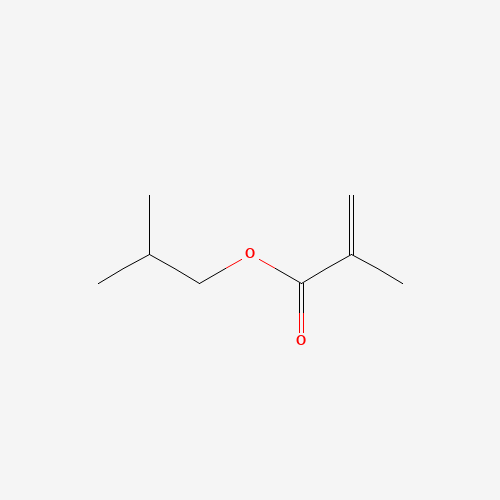
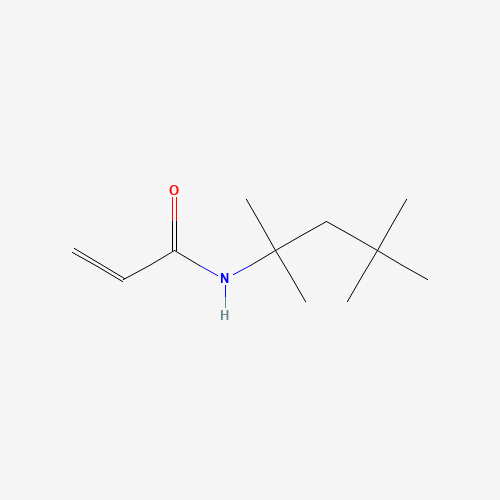
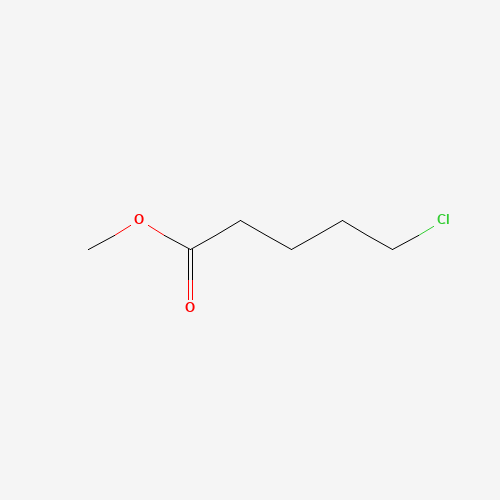
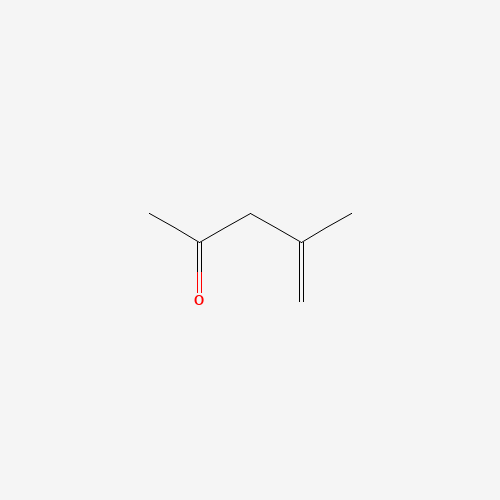
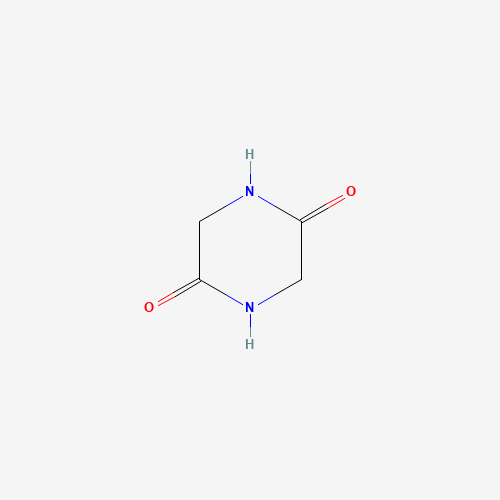
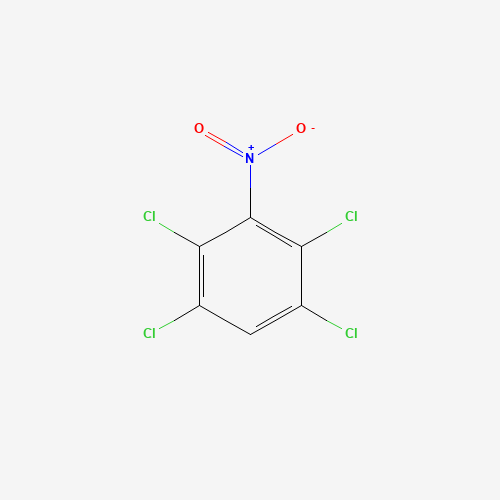
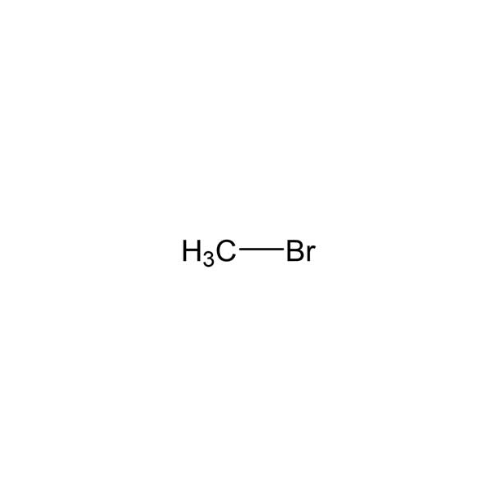
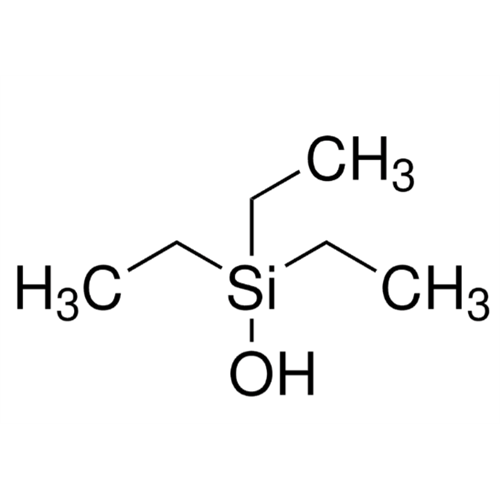
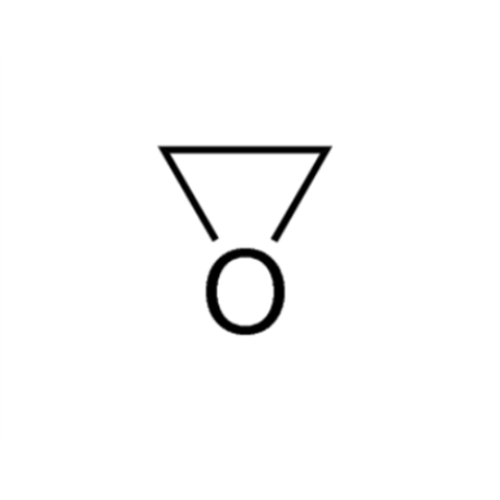
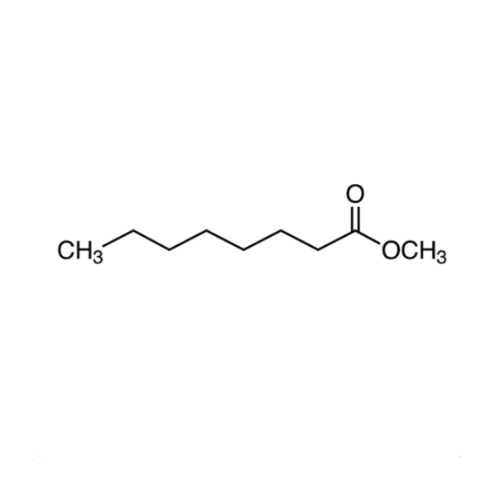
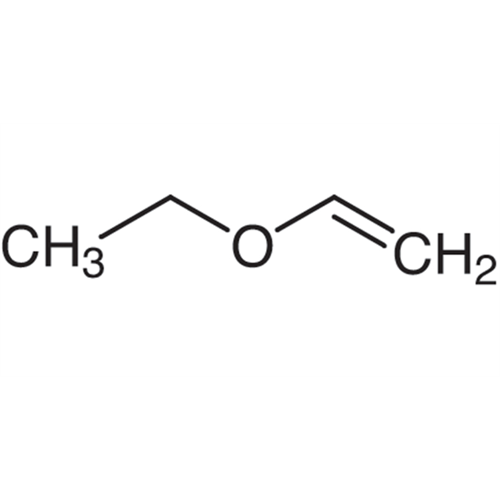
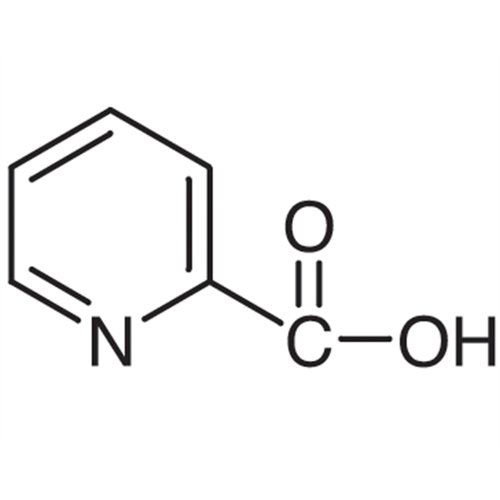
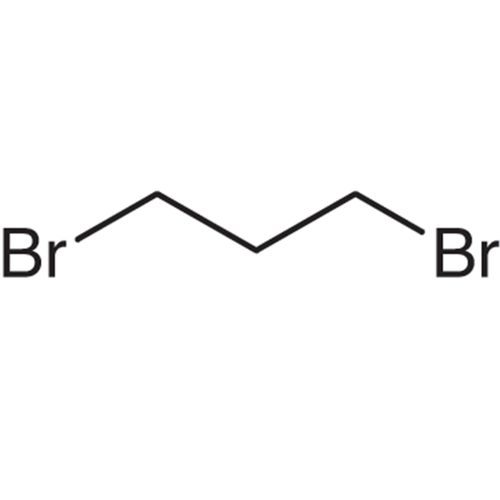
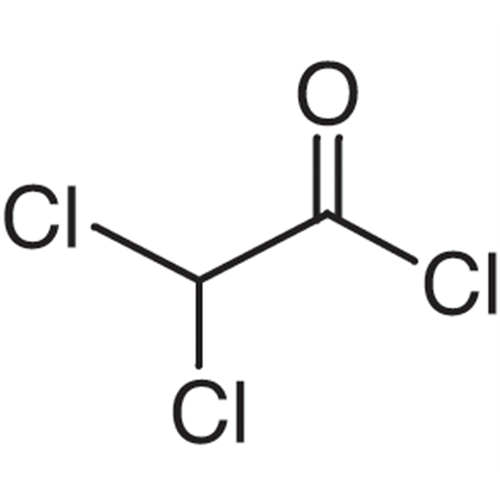
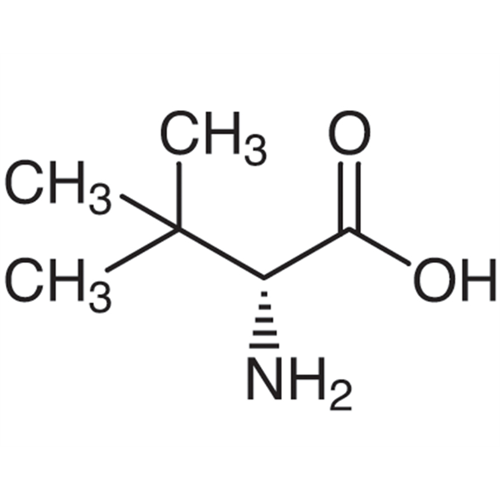
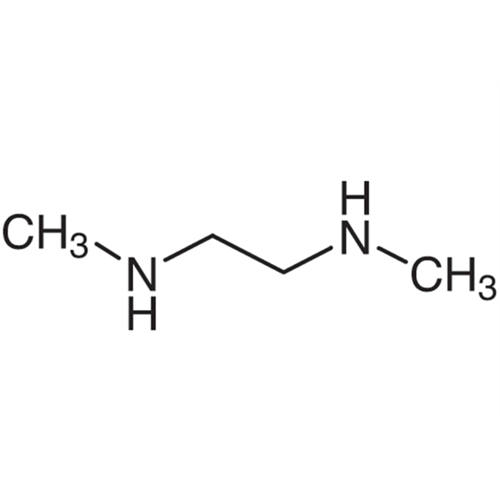
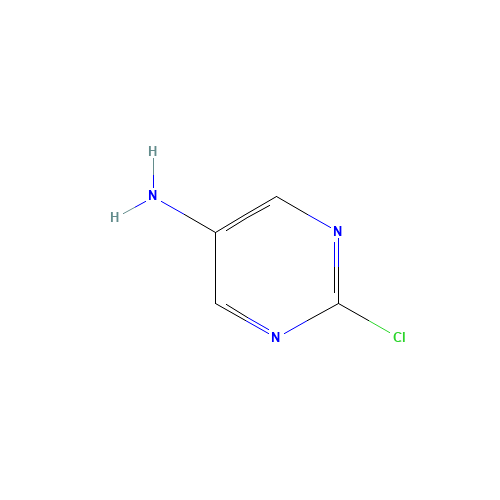
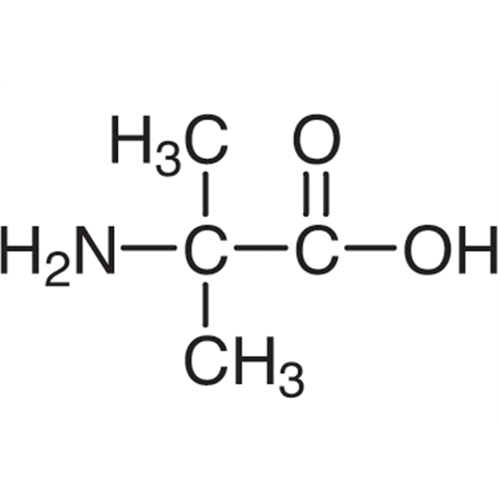
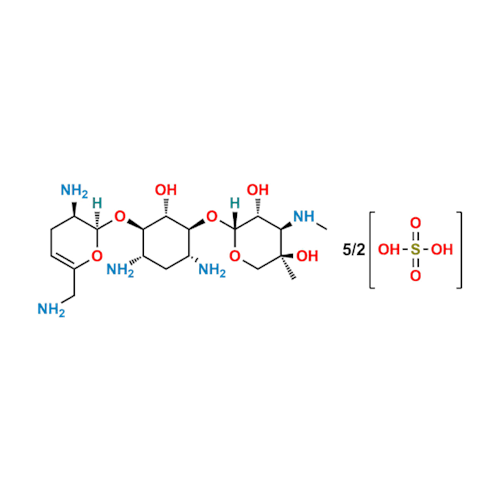
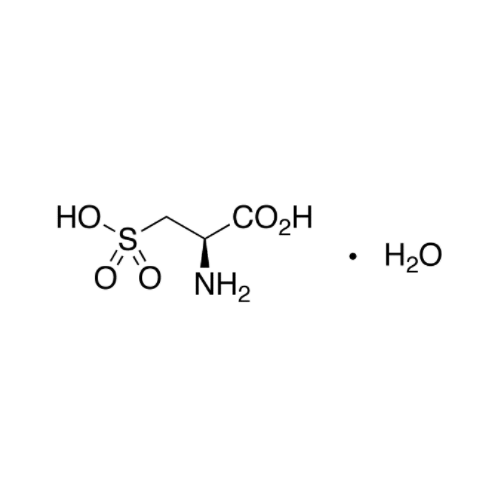
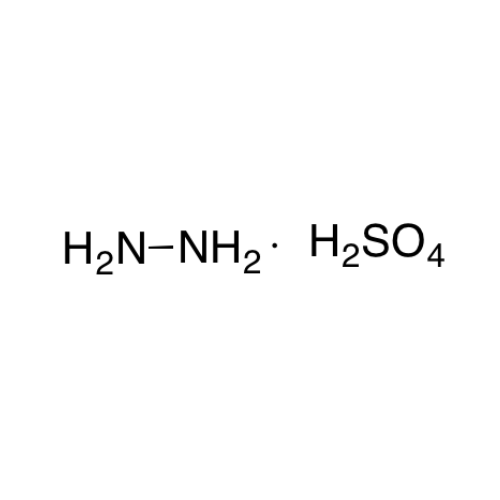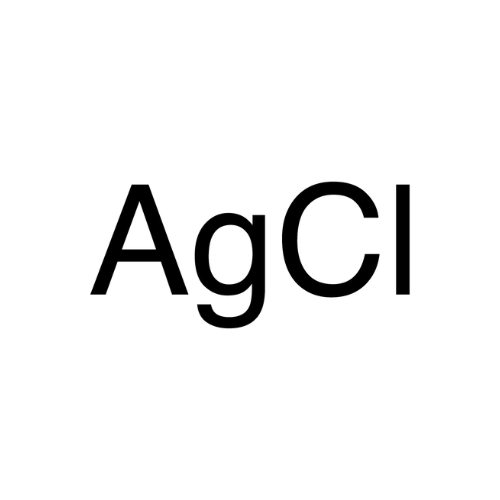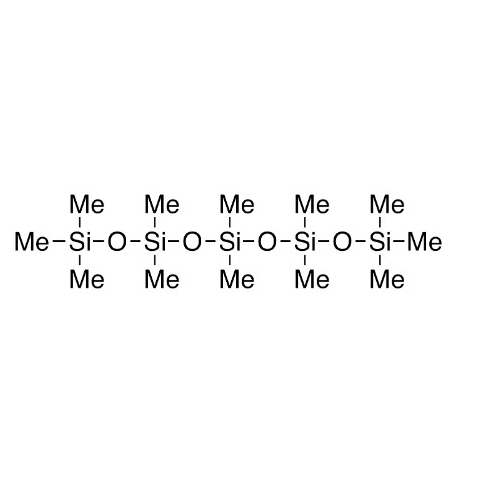Sisomicin Sulfate Analytical Standards
Add to Cart
Bulk Enquiry
|
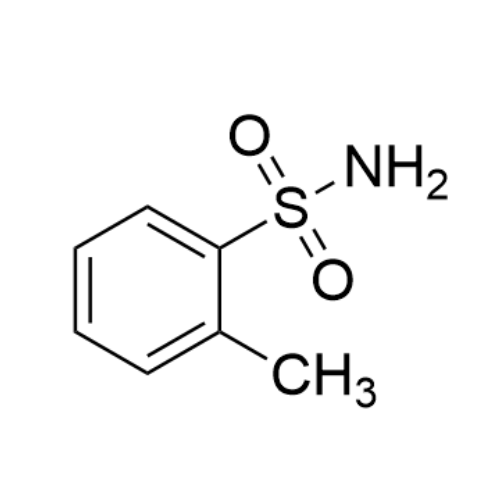
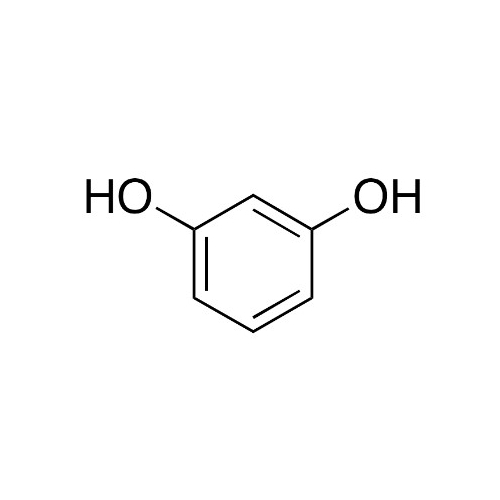
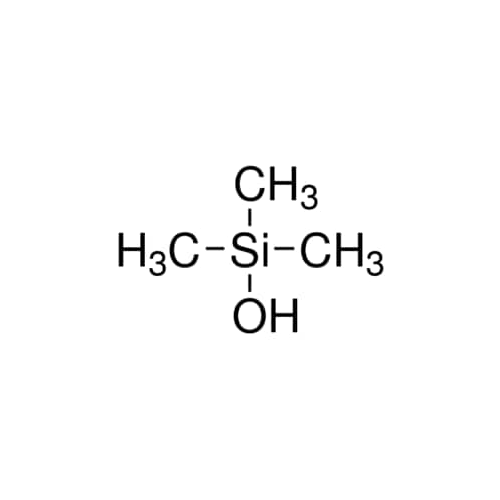
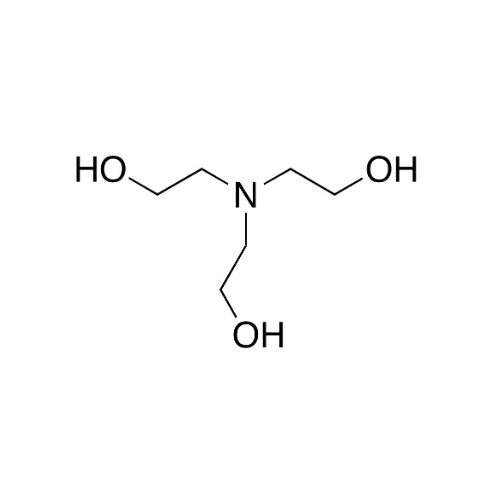
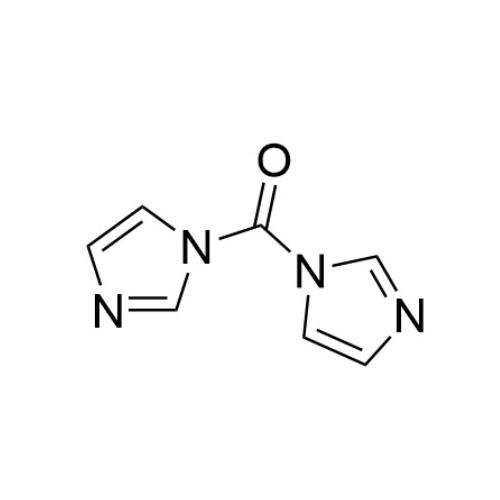
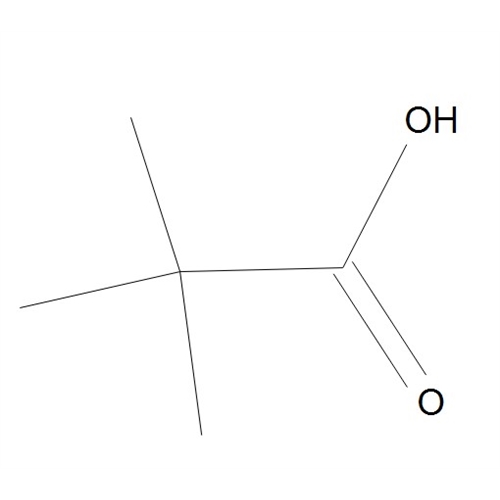
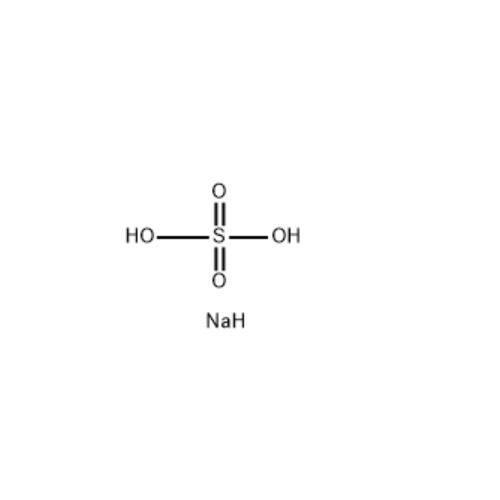
Chemical Name: Sisomicin Sulfate Analytical Standards
Synonym: Sisomicin Sulfate


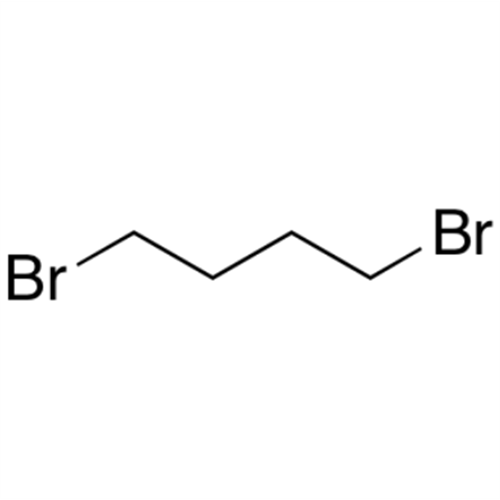
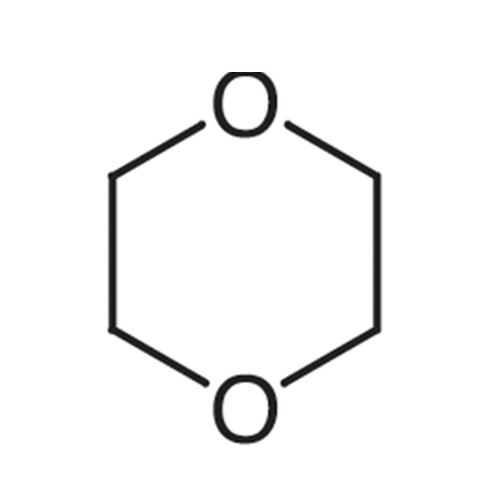
![1,8-Diazabicyclo(5.4.0)undec-7-ene [DBU] GC STANDARD 1,8-Diazabicyclo(5.4.0)undec-7-ene [DBU] GC STANDARD](/uploads/product-details/gcs018-682.png)
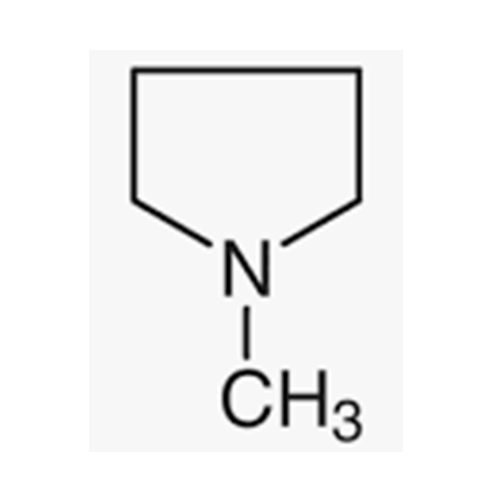
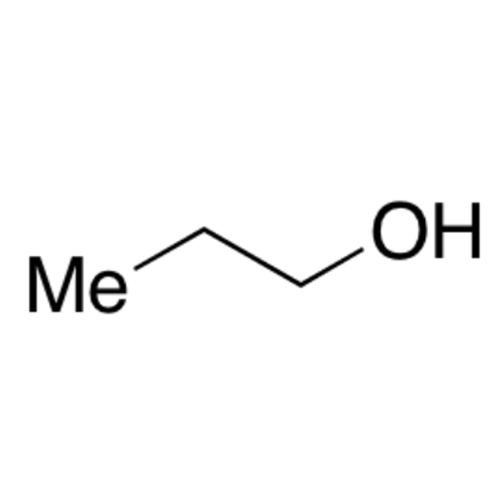
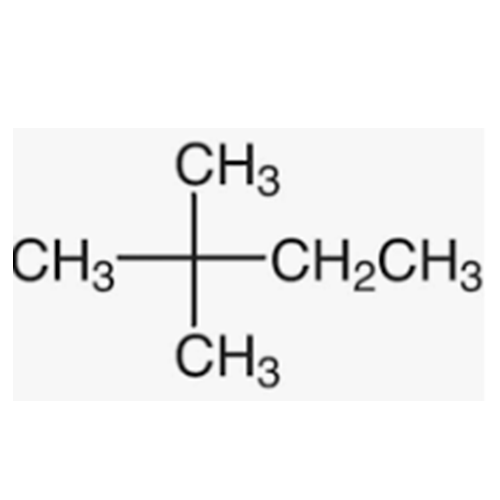
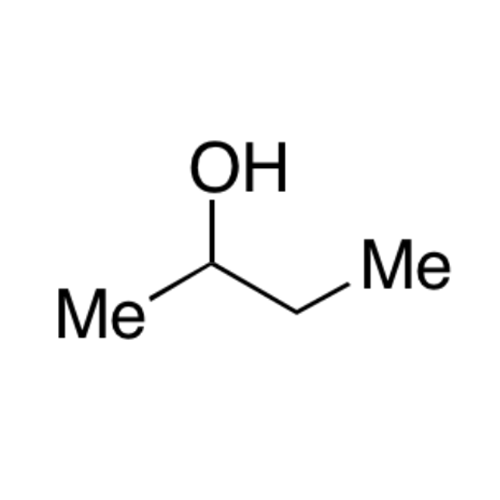
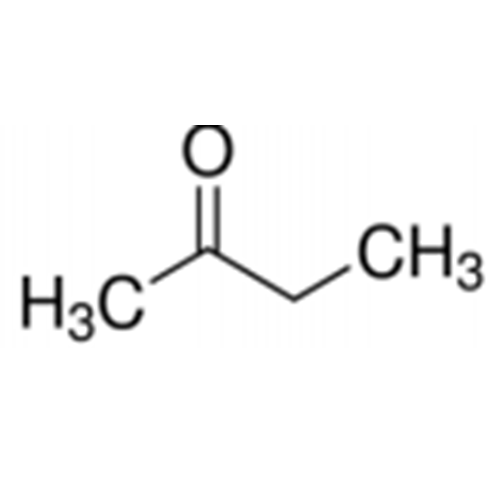
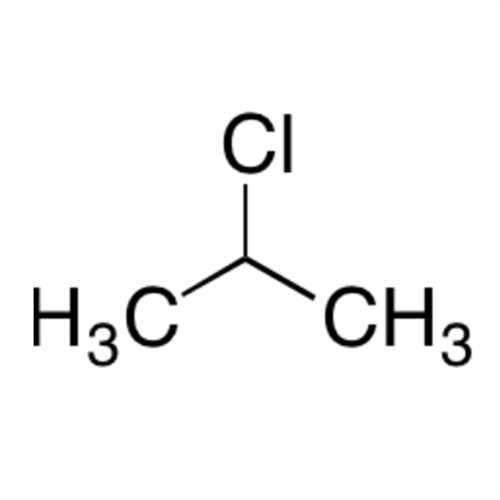
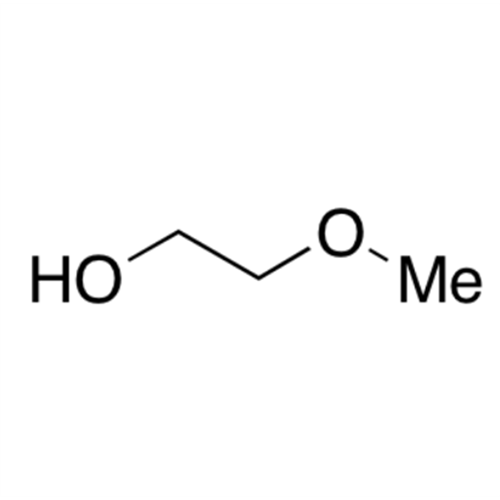
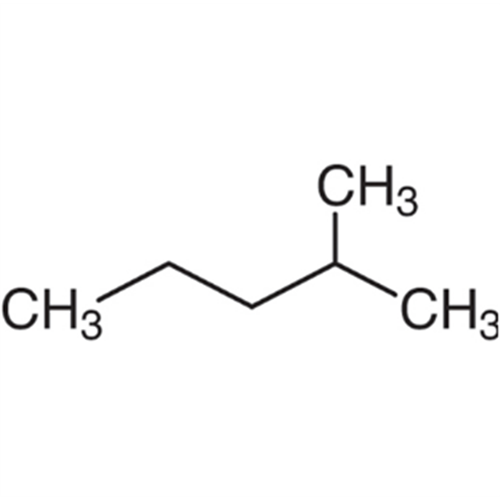
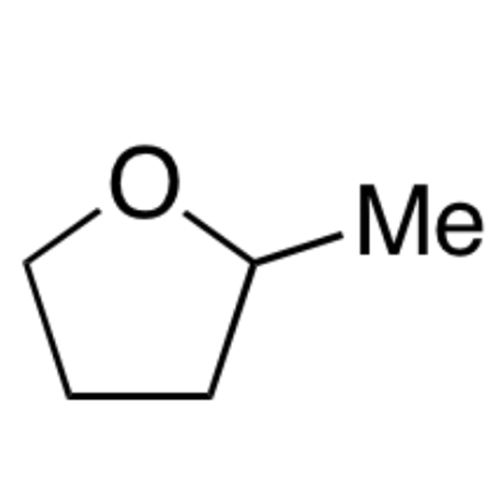
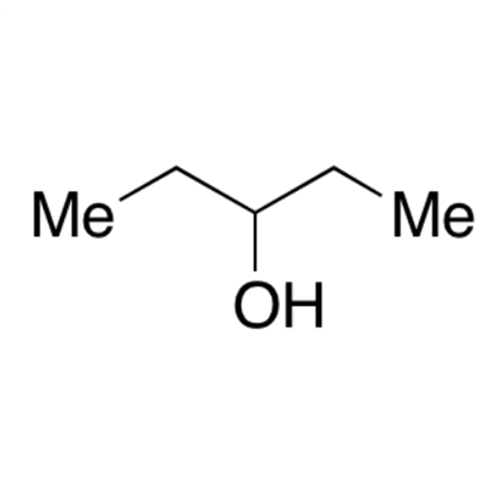
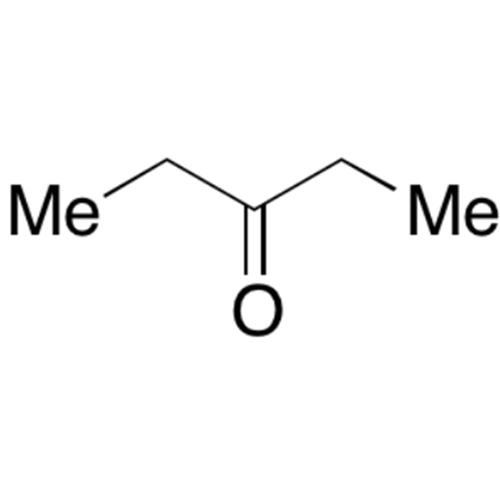
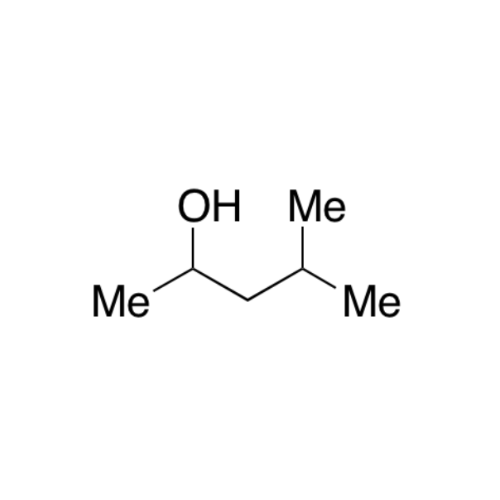
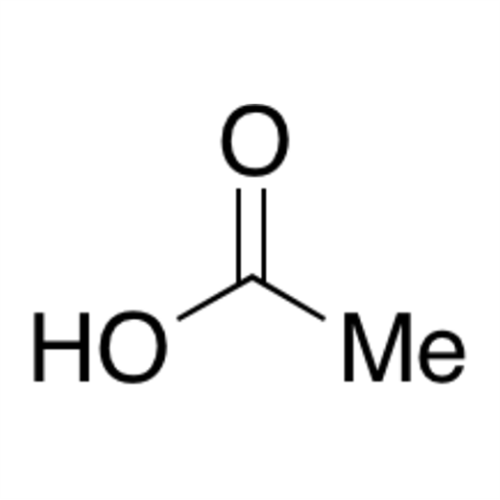
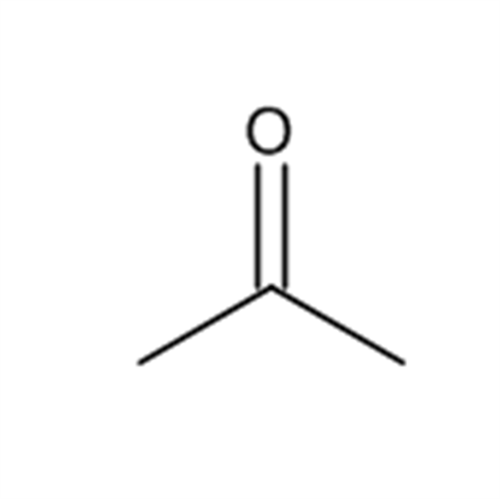
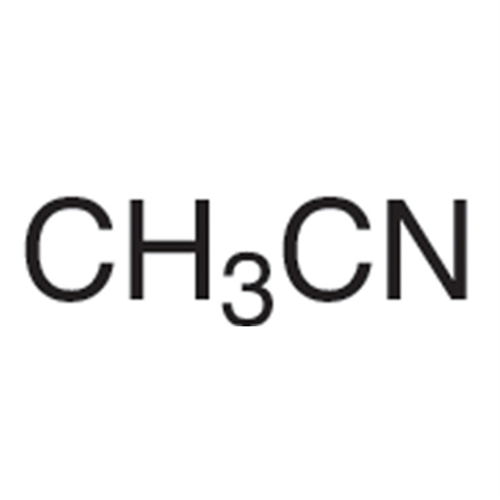
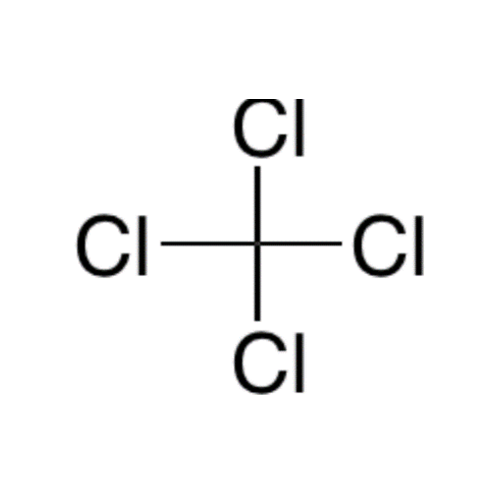
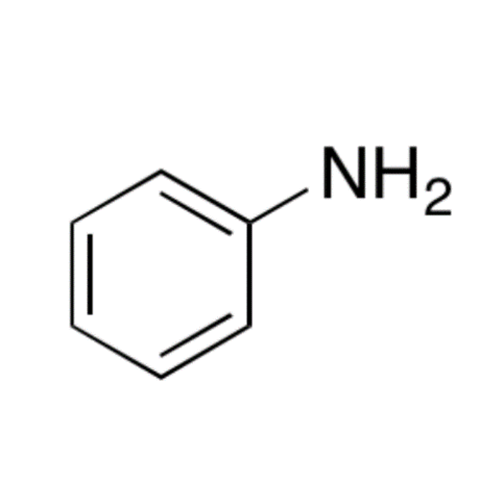
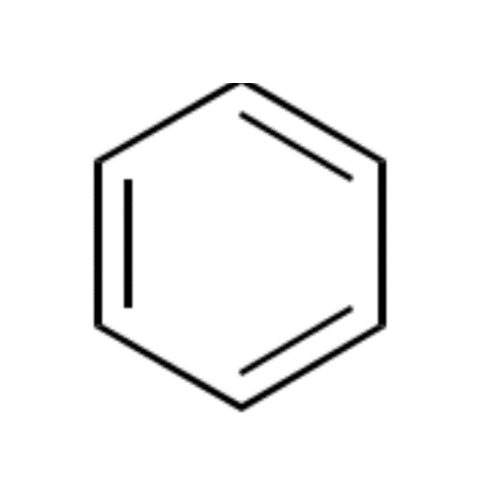
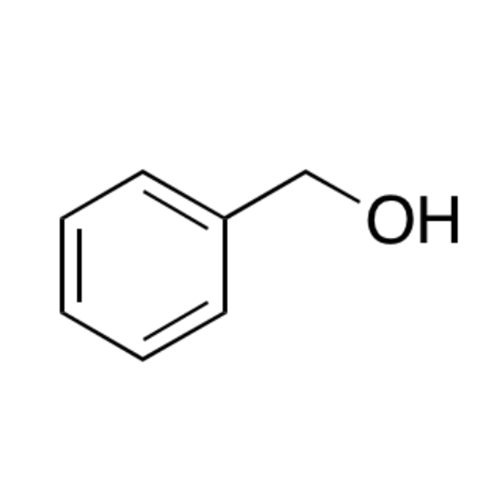
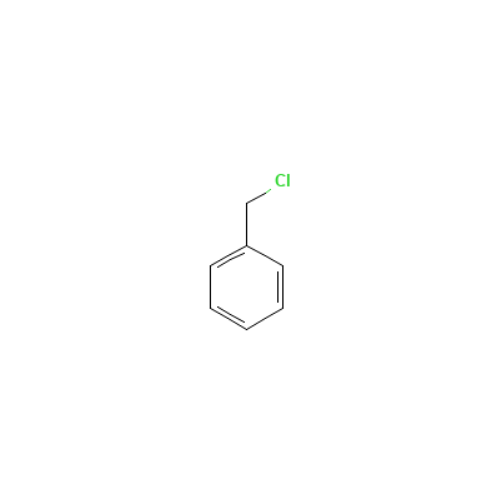
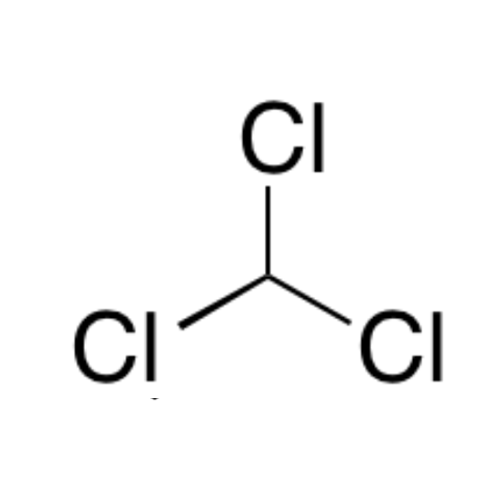
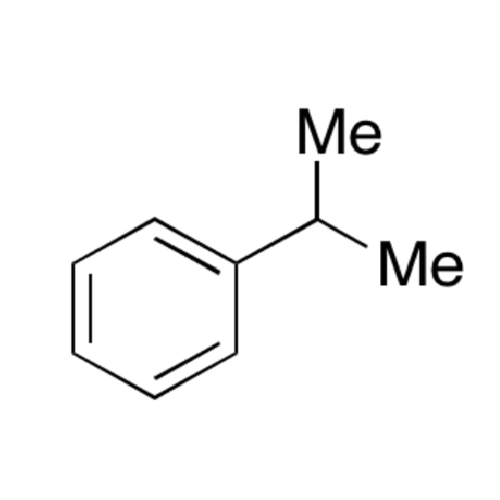
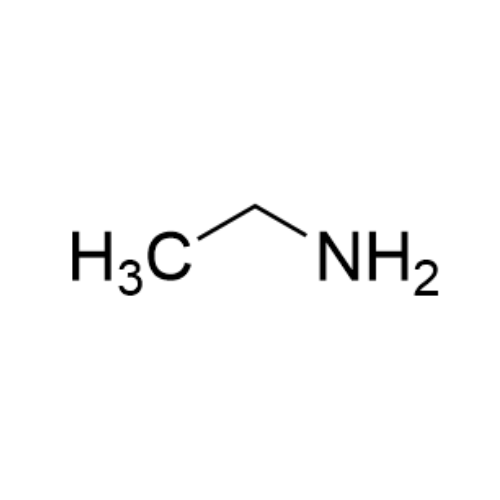
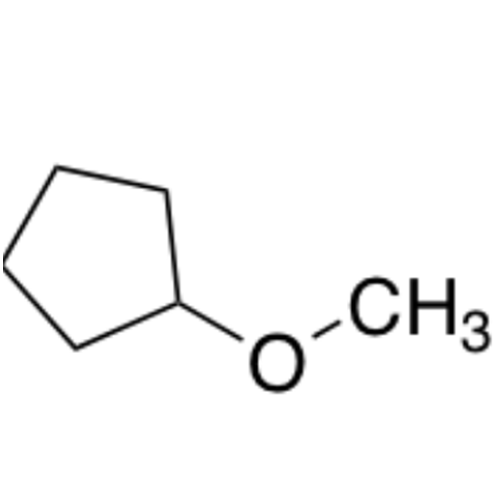
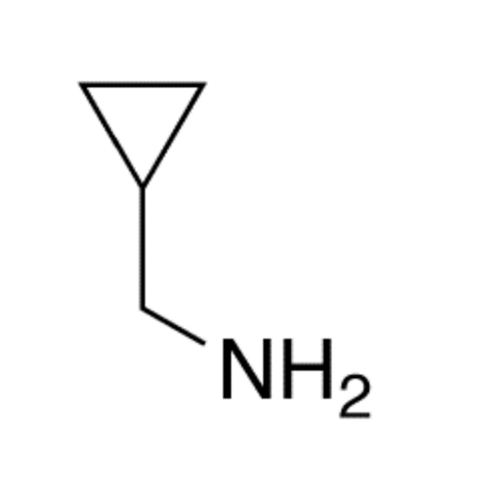
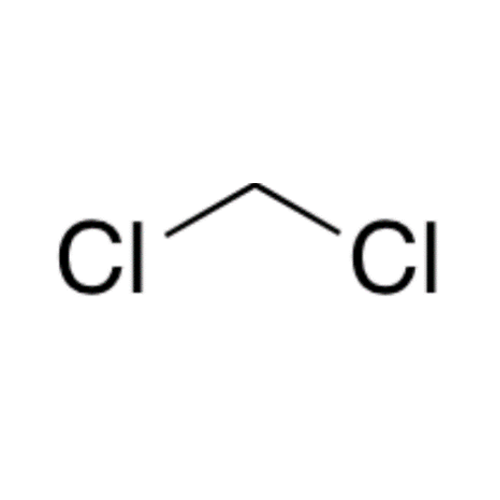
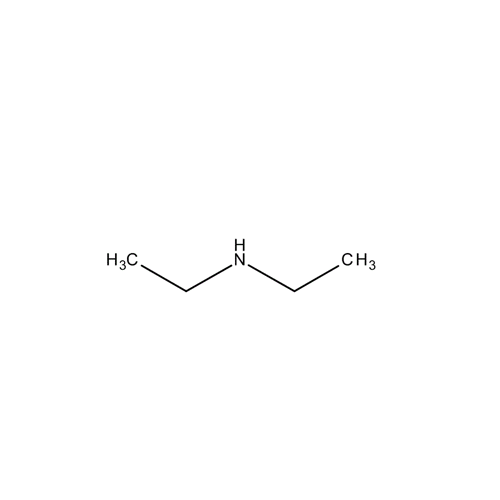
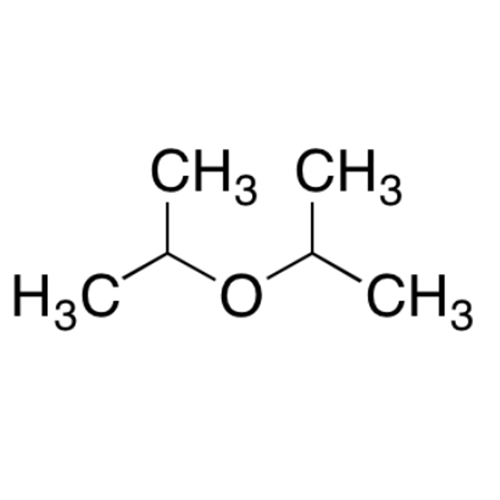
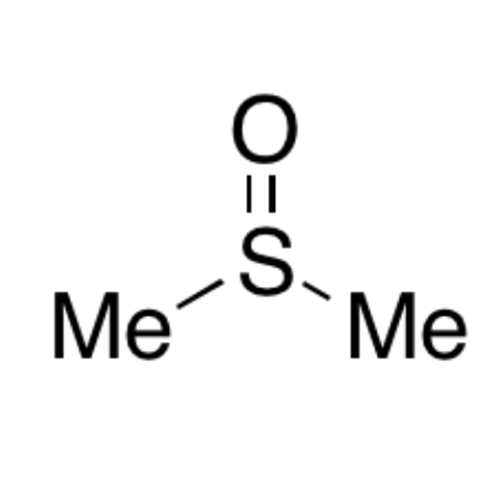
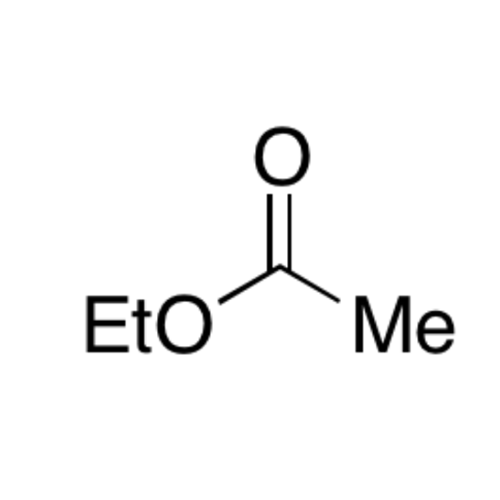
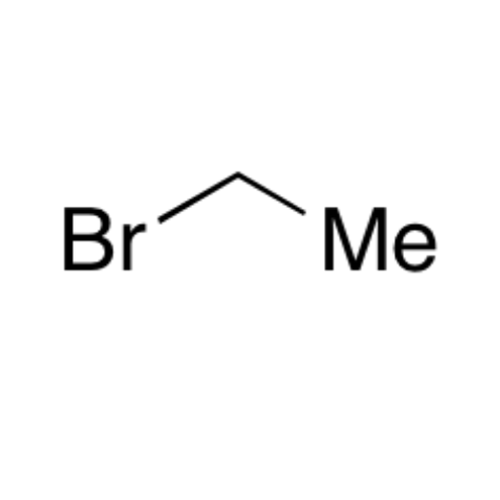
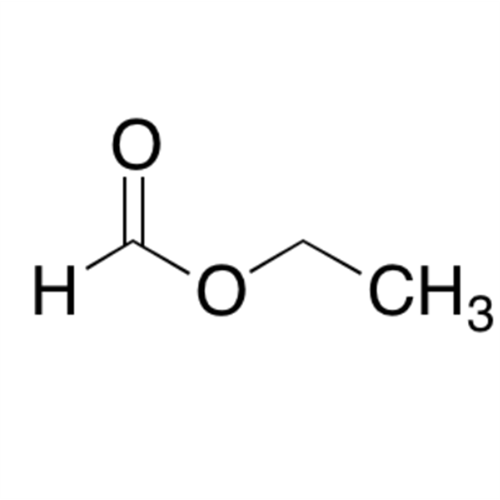
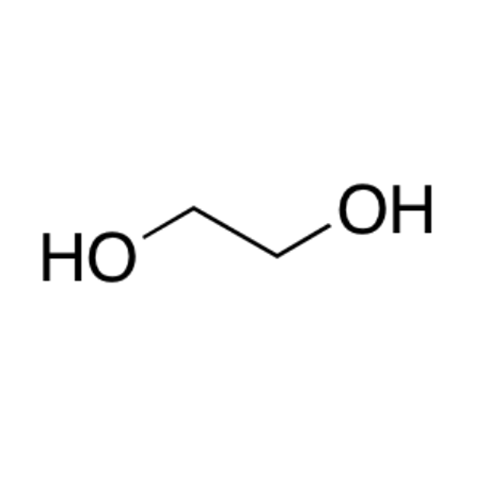
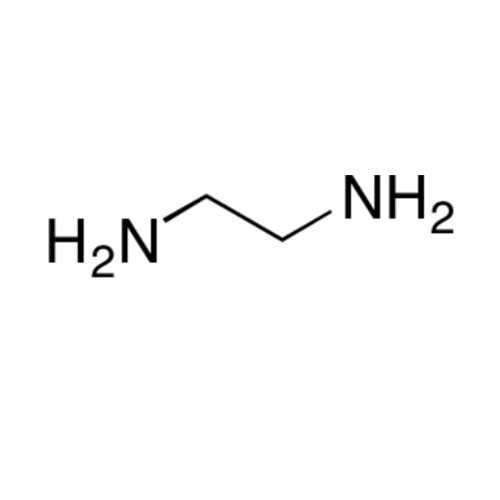
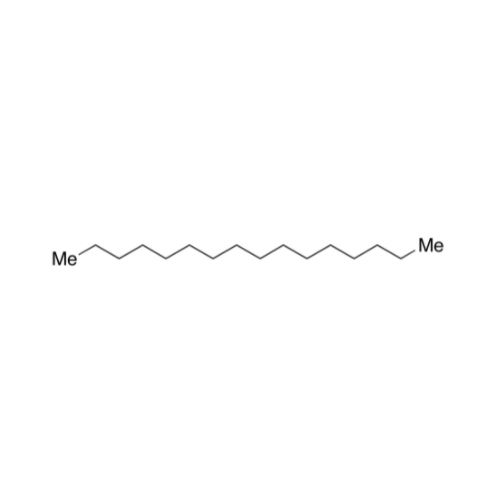
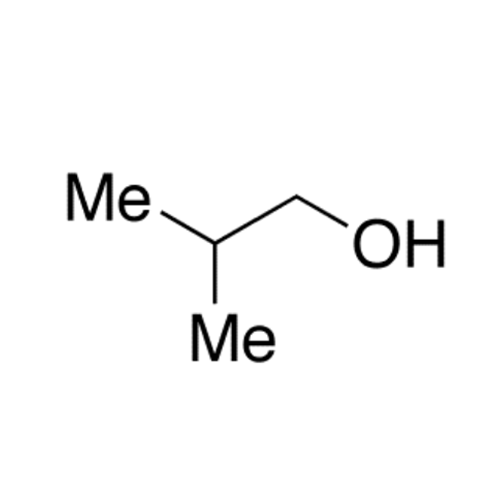
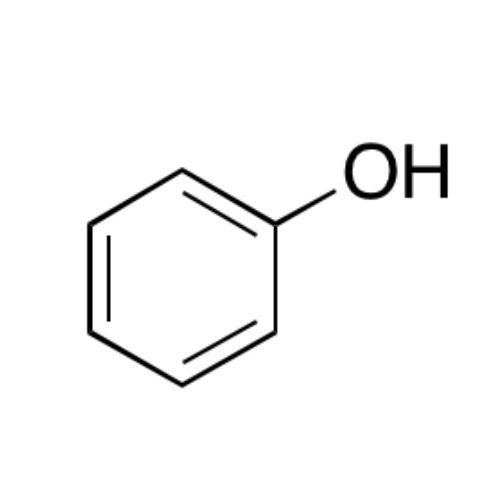
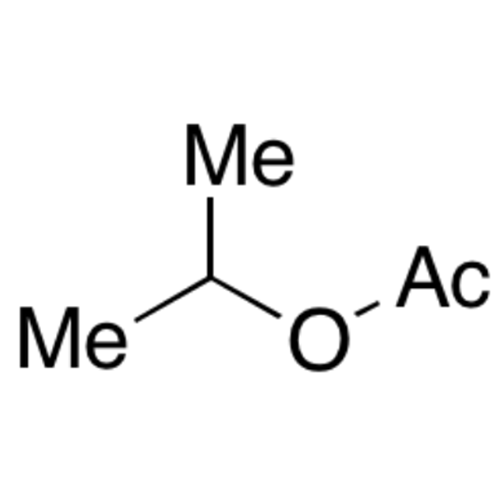
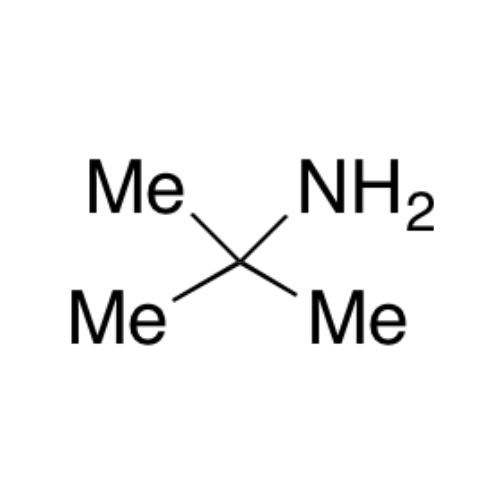
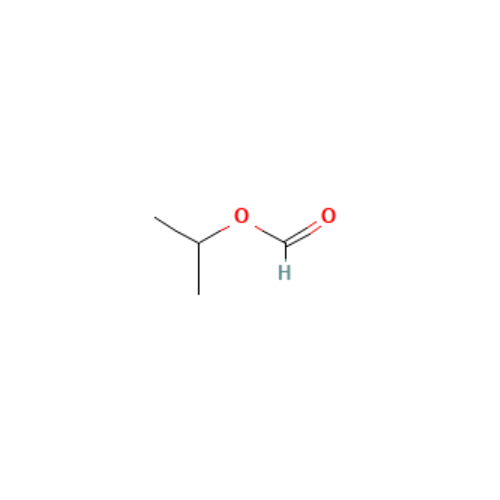
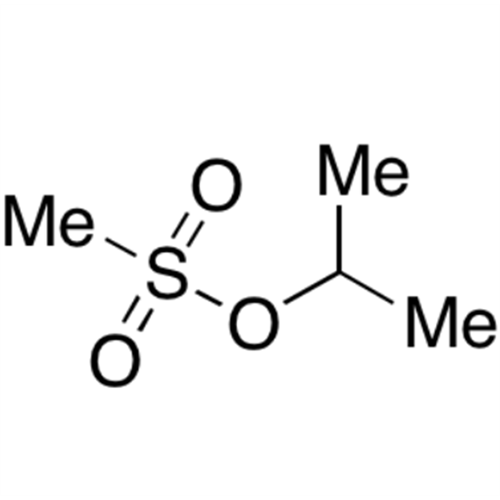
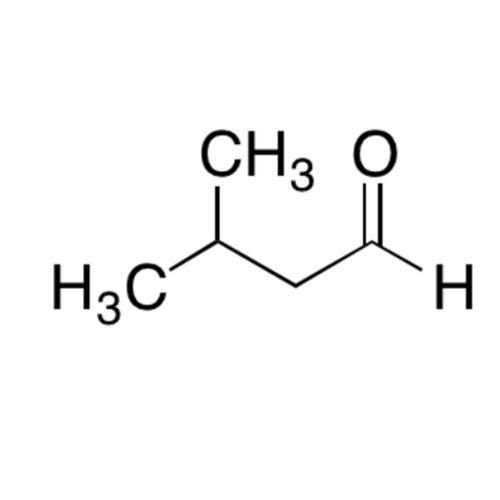
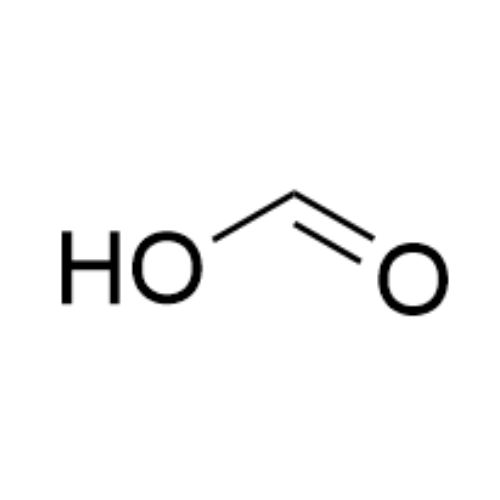
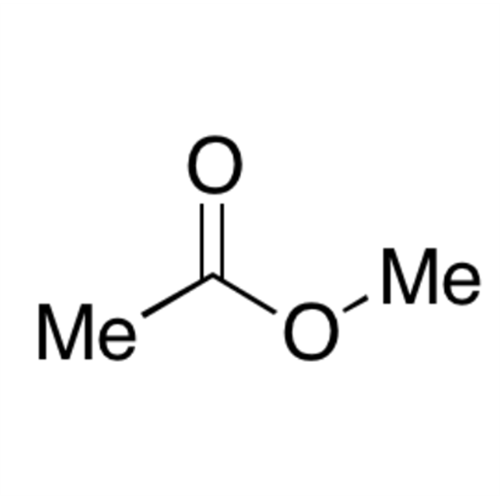
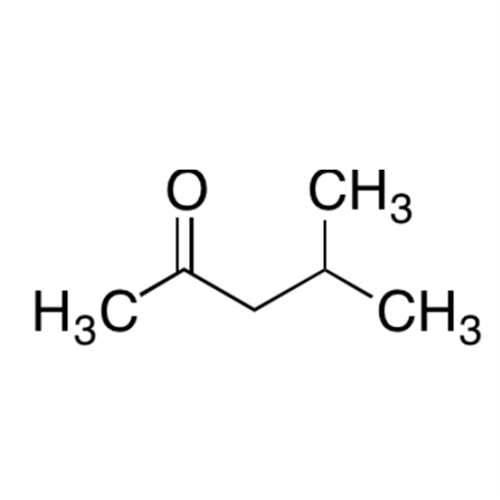
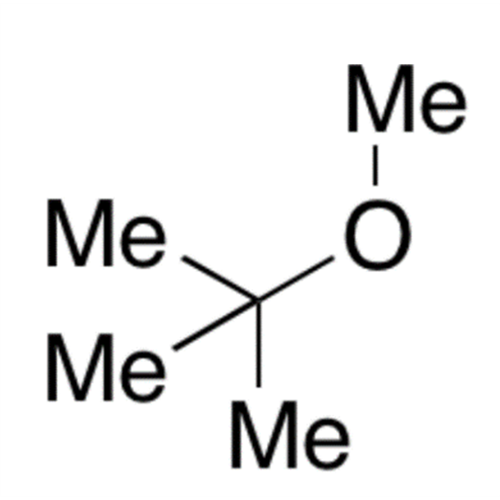
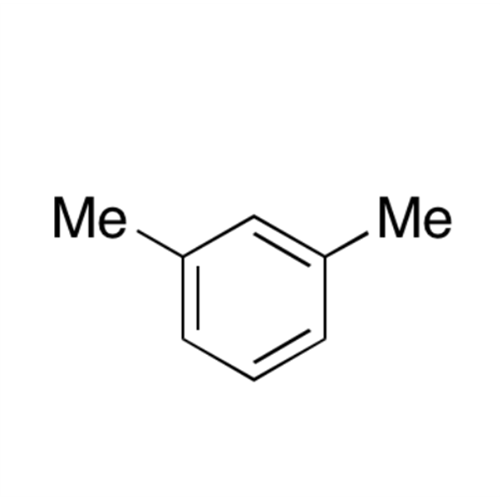
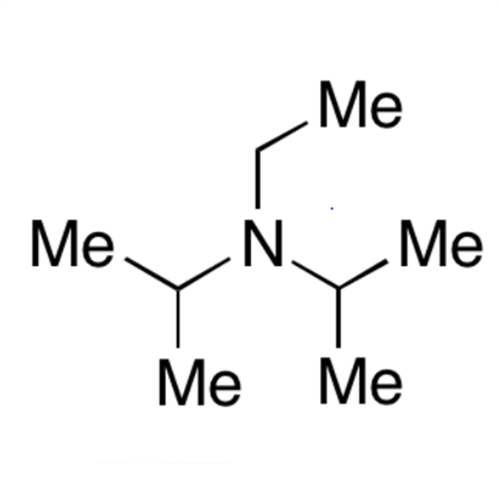
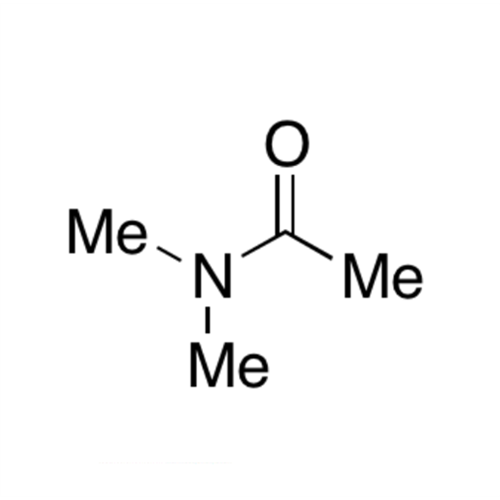
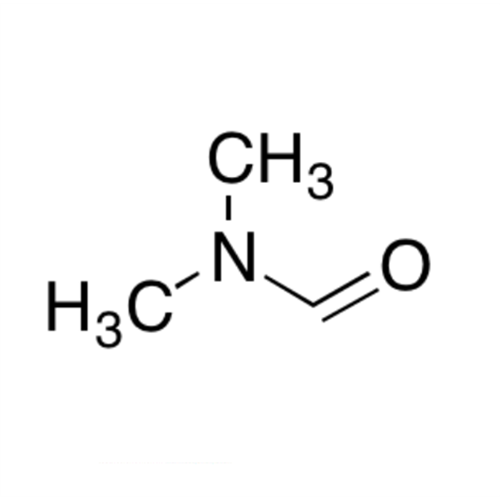
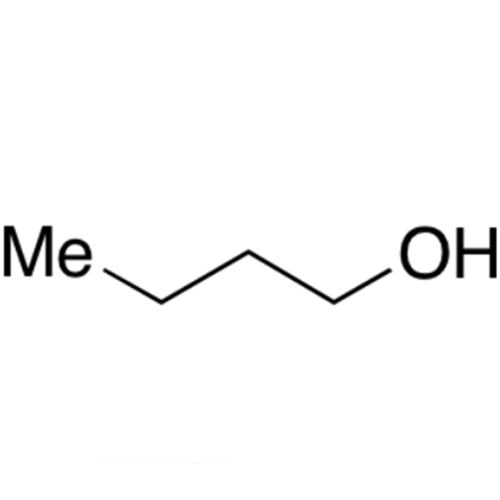
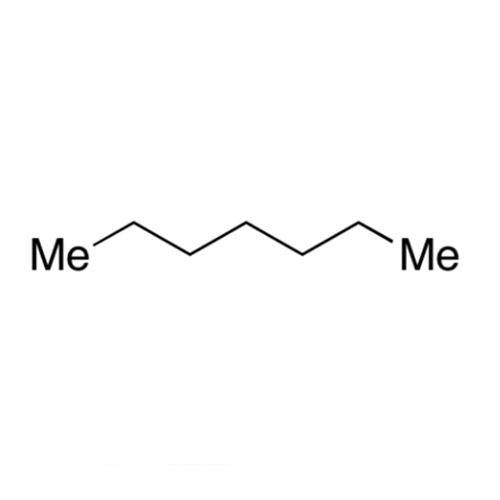
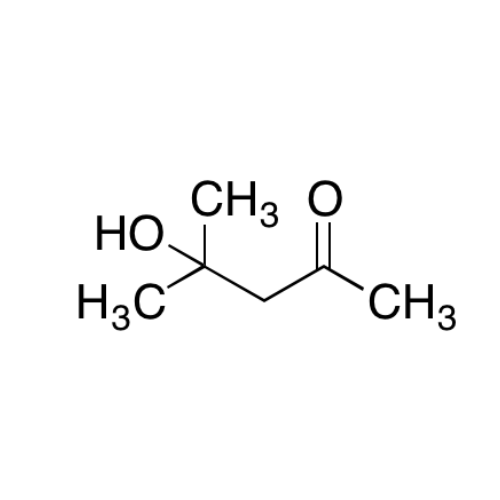
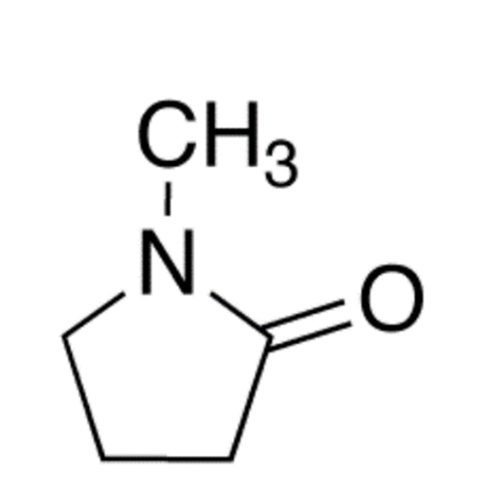

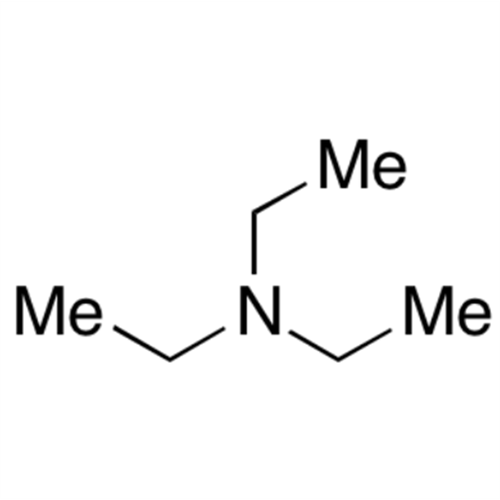
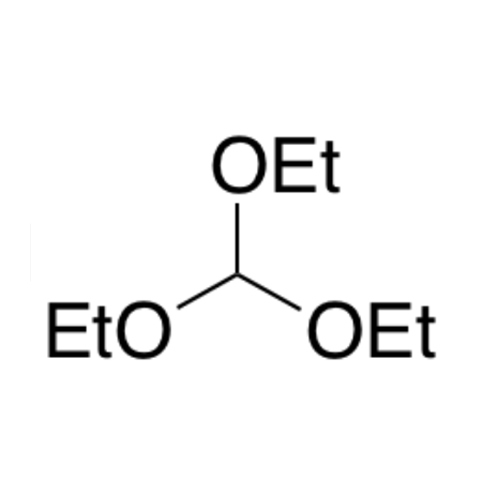
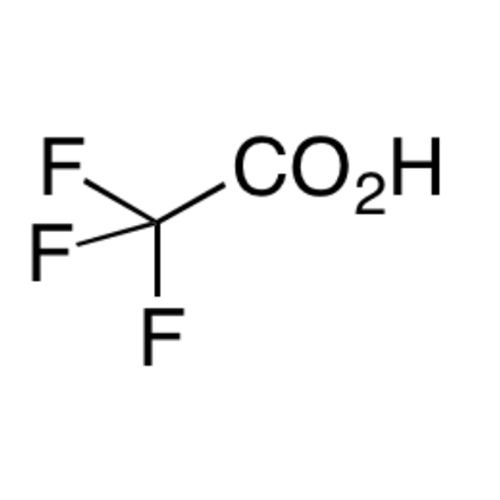
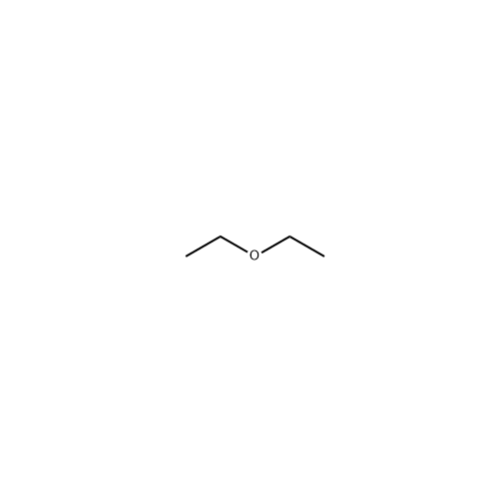
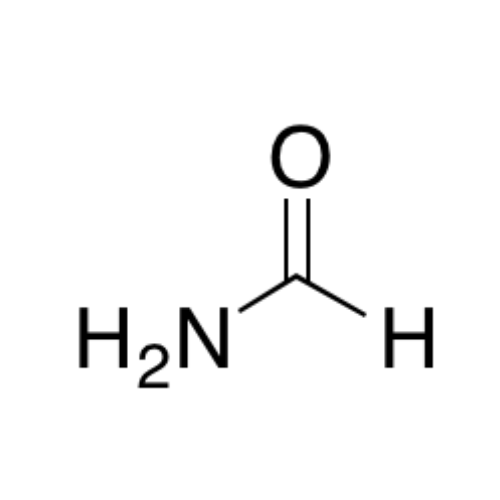
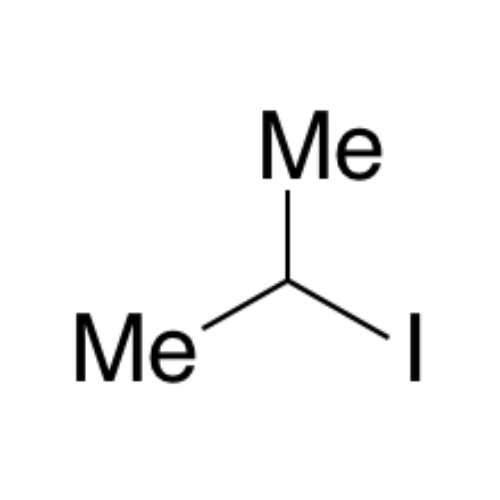
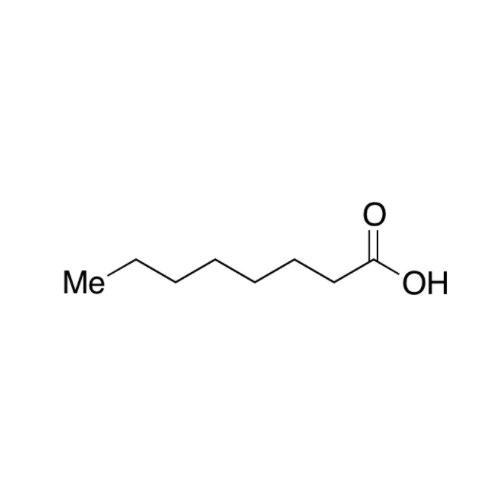
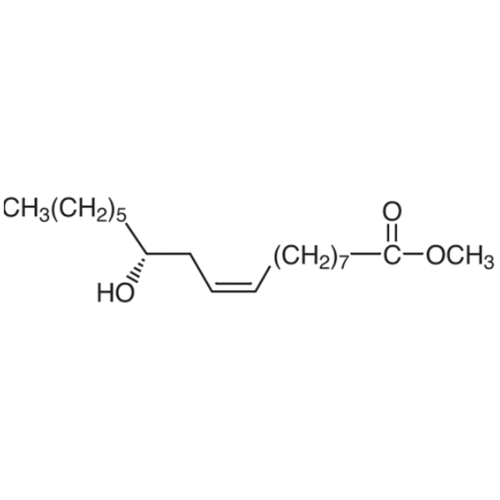
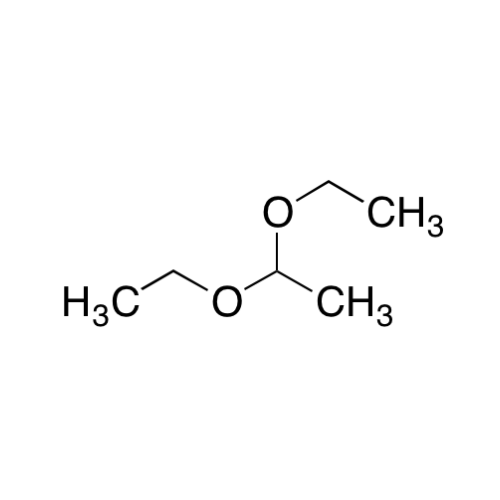
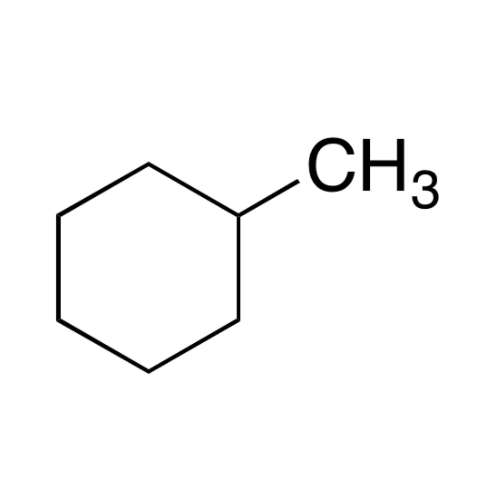
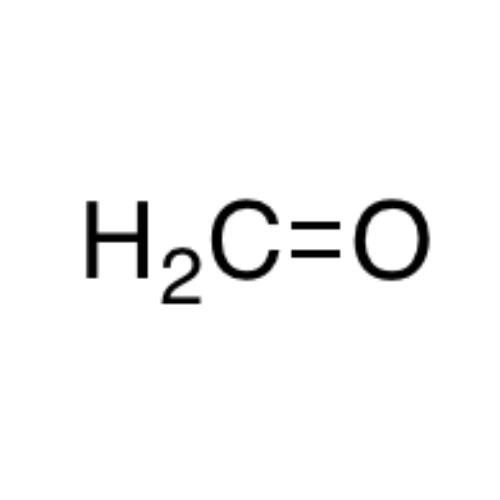
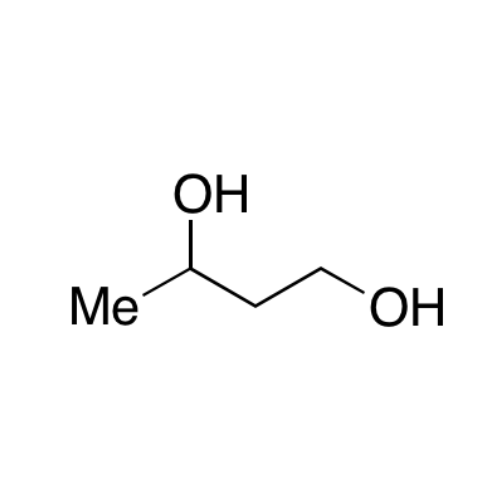
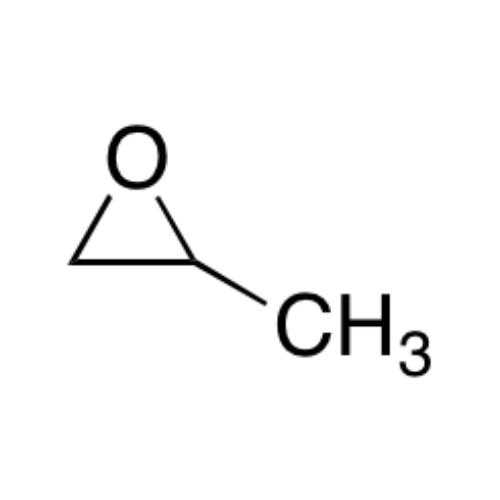
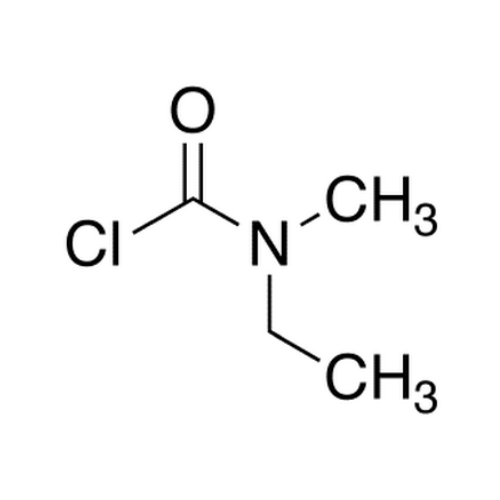
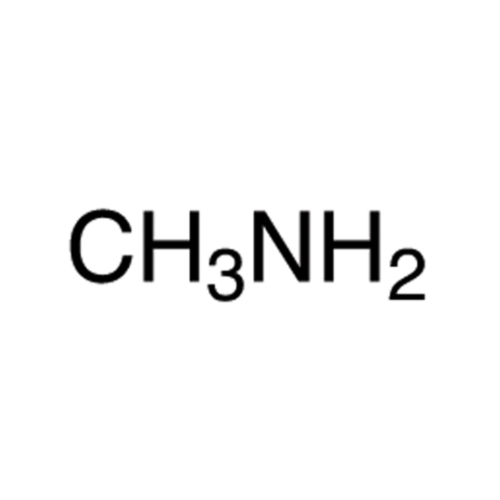
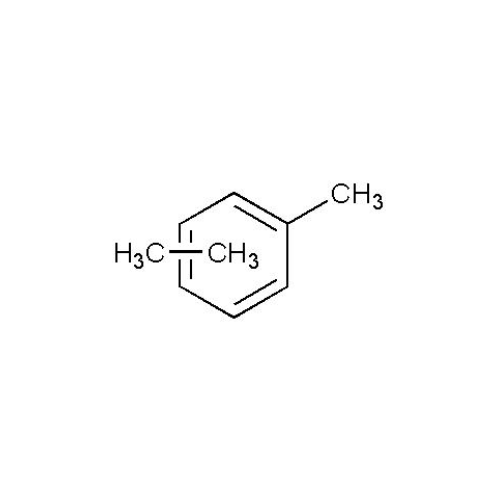
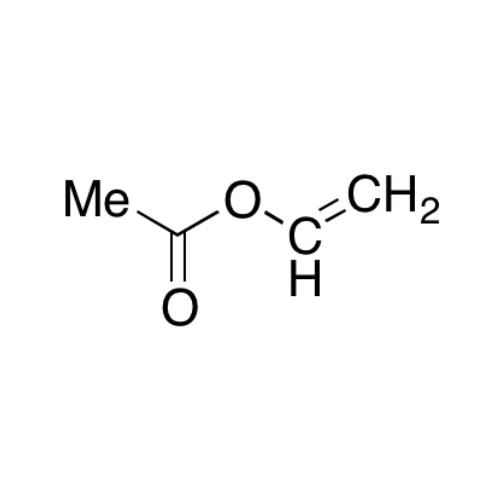
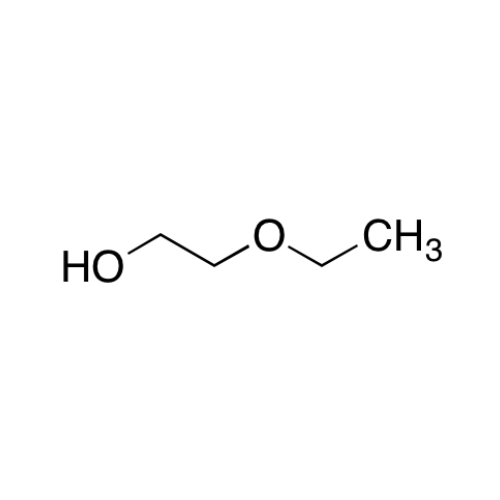
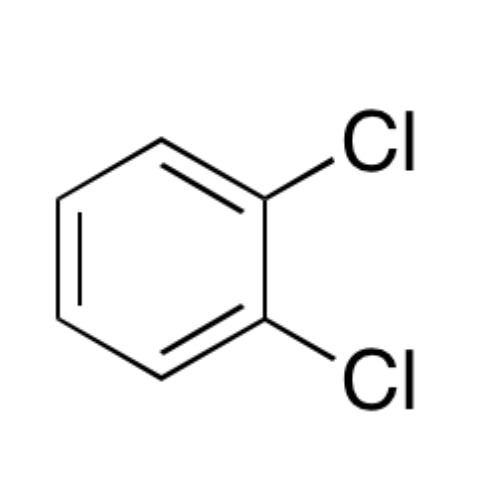
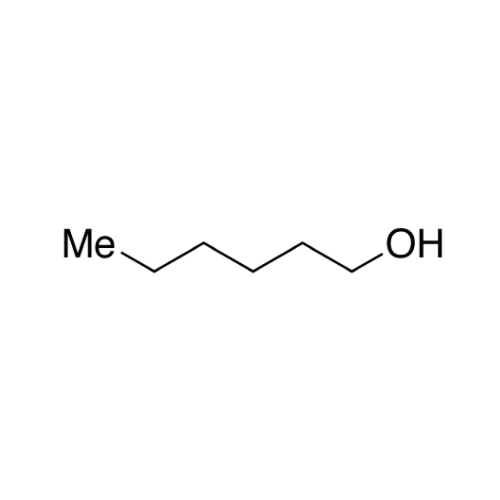
![2-[2-(2-Chloroethoxy)ethoxy]ethanol GC Standard 2-[2-(2-Chloroethoxy)ethoxy]ethanol GC Standard](/uploads/product-details/gcs118-1422.png)