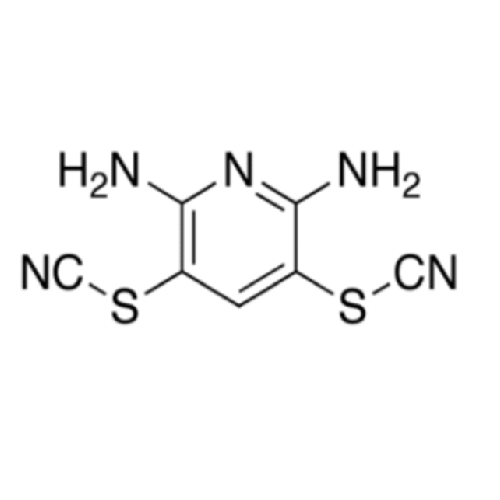
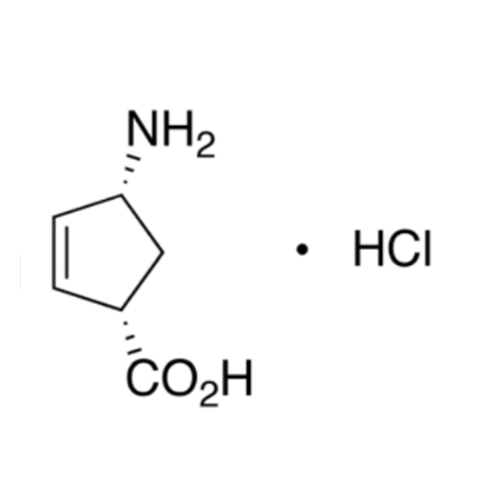
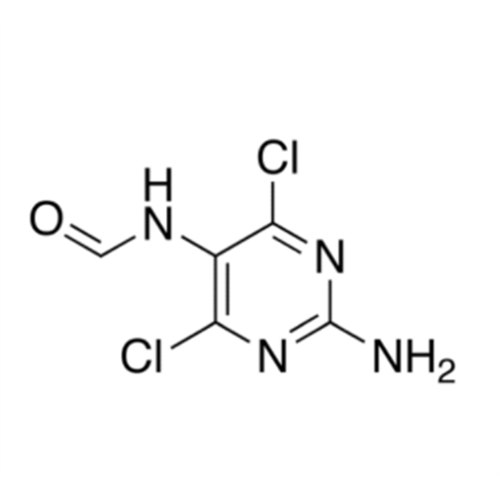
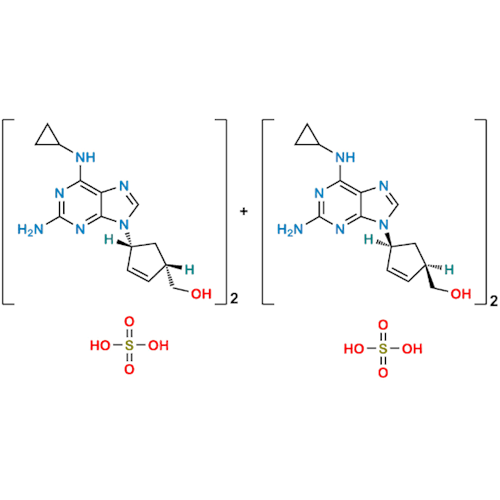
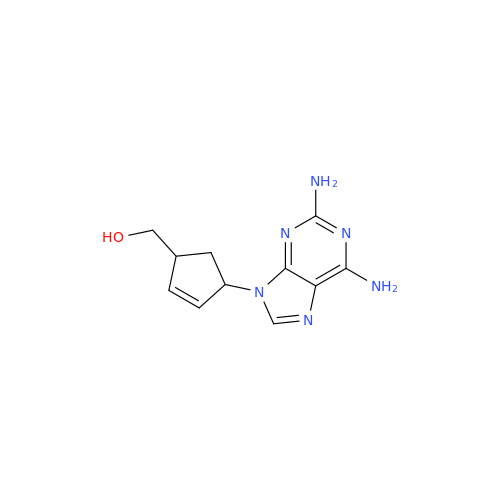
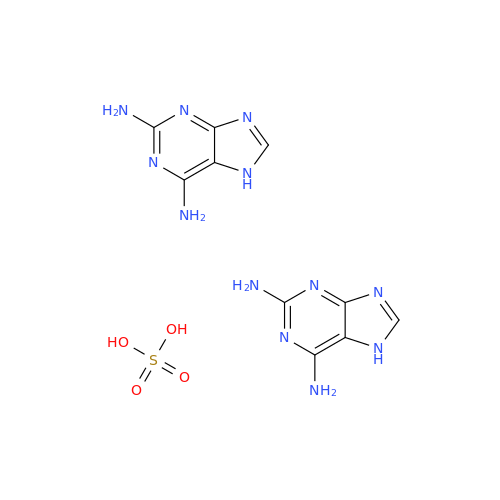
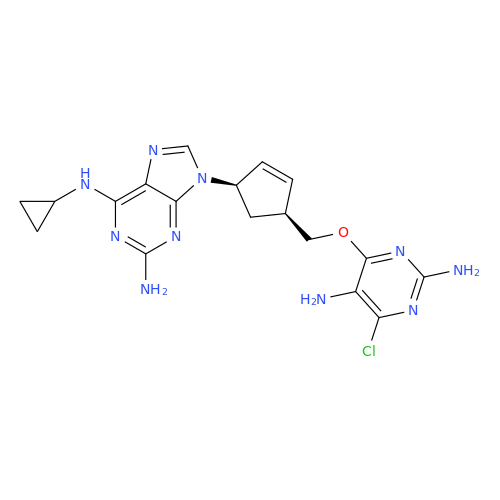
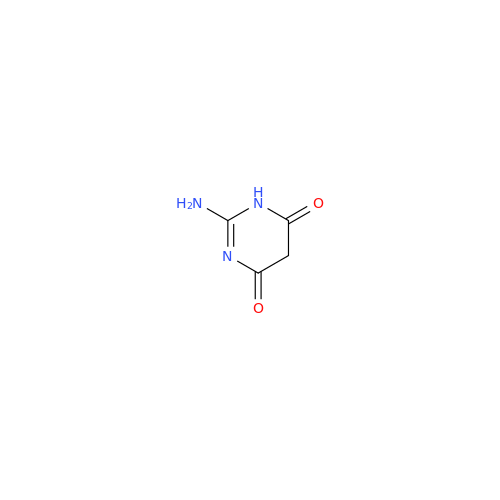
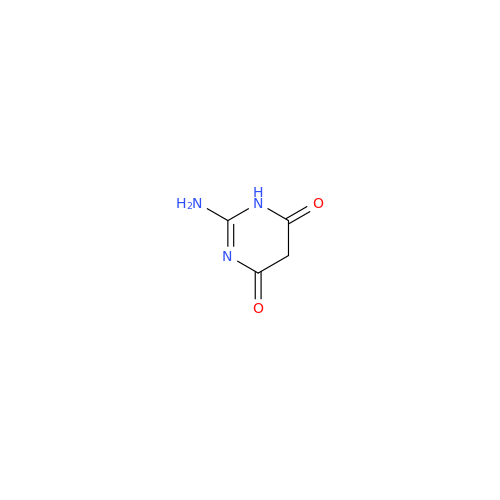
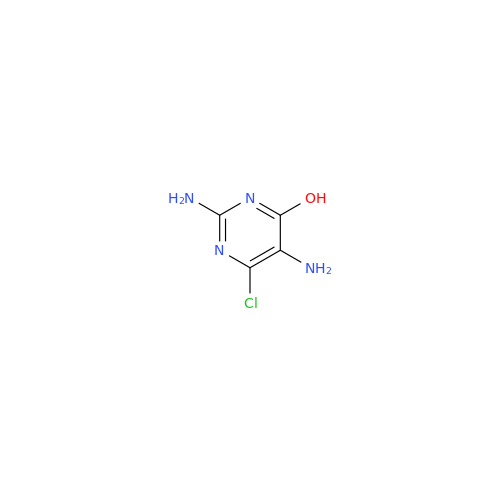
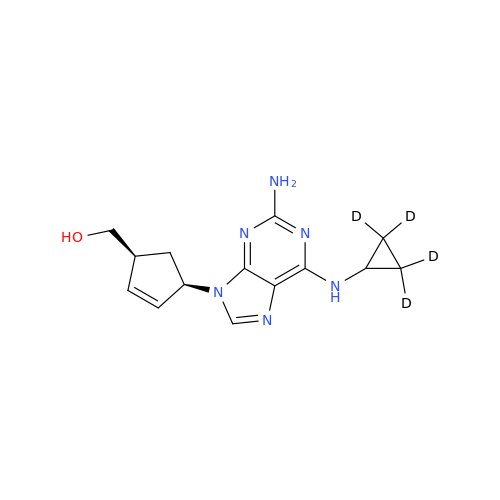
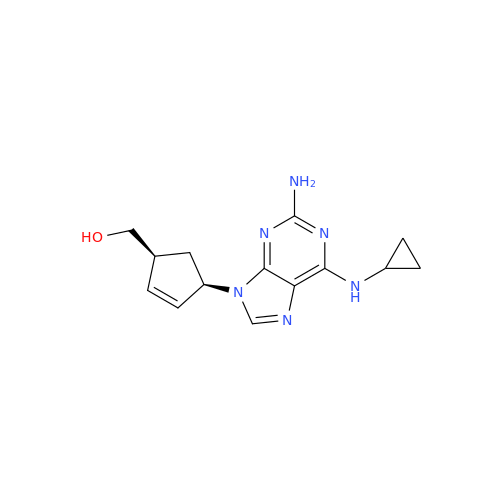
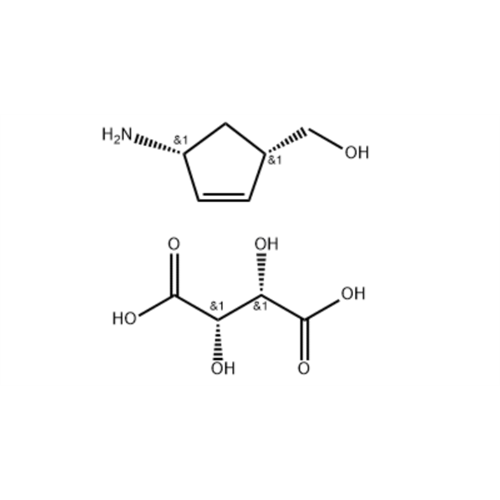
Product Information
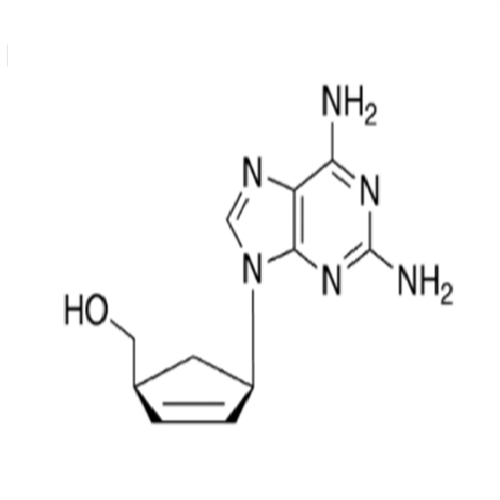
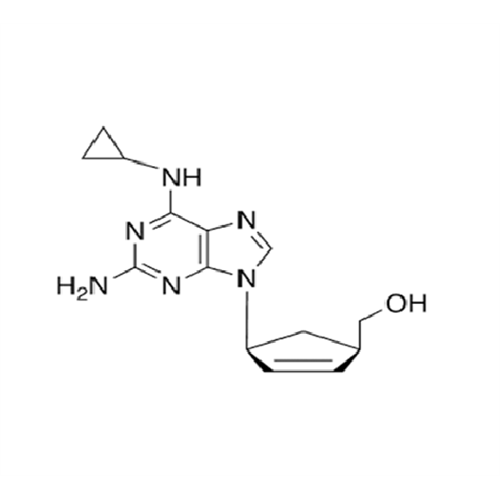
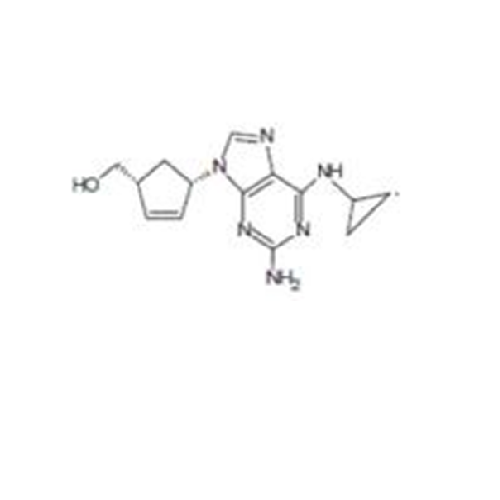
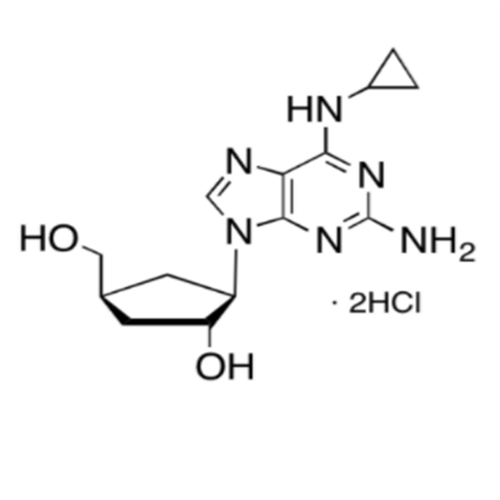
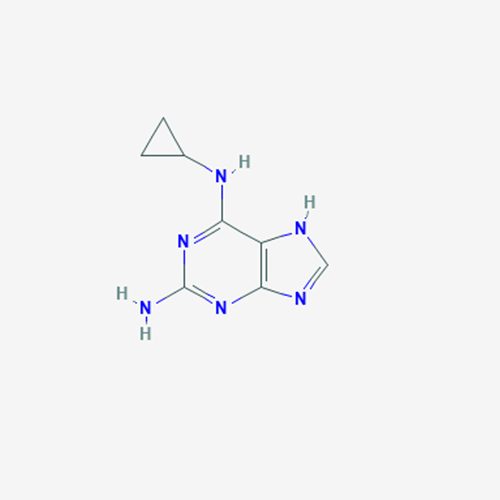
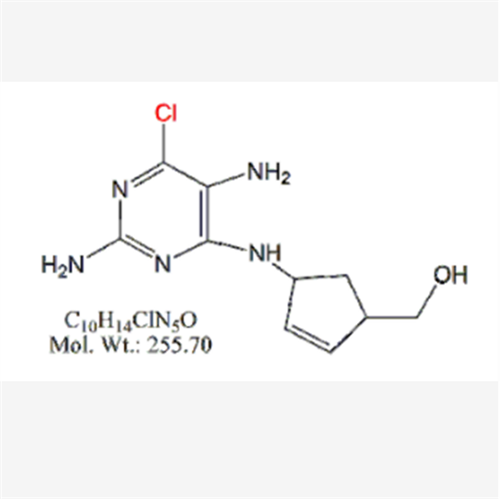
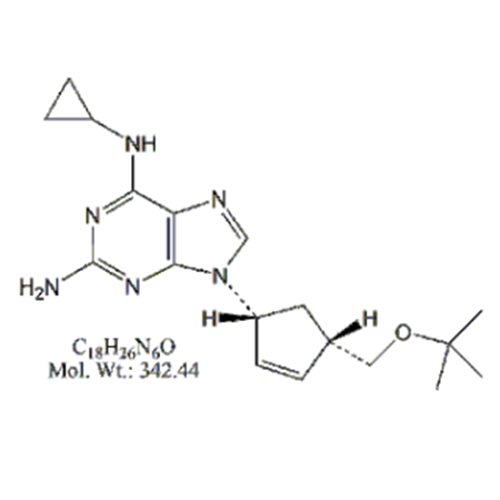
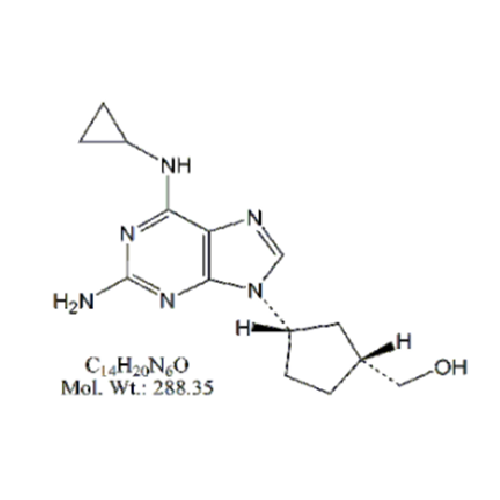
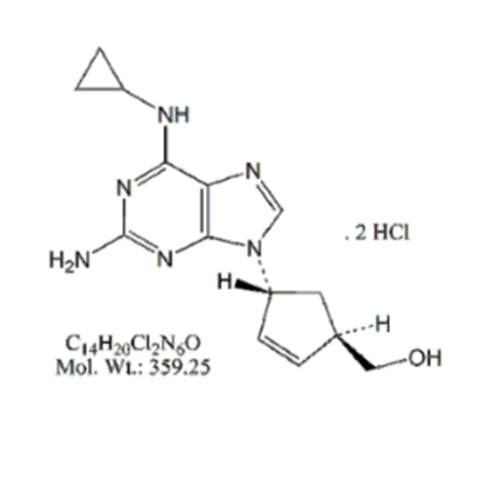
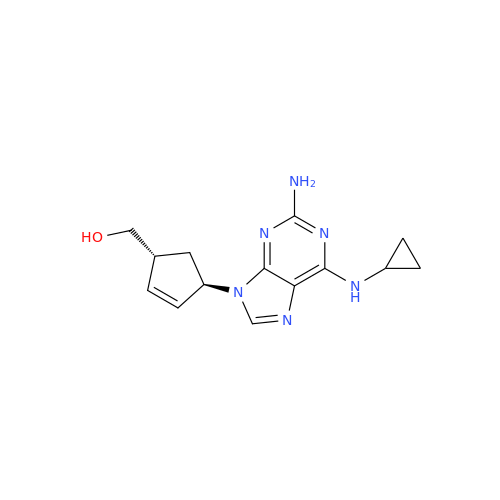
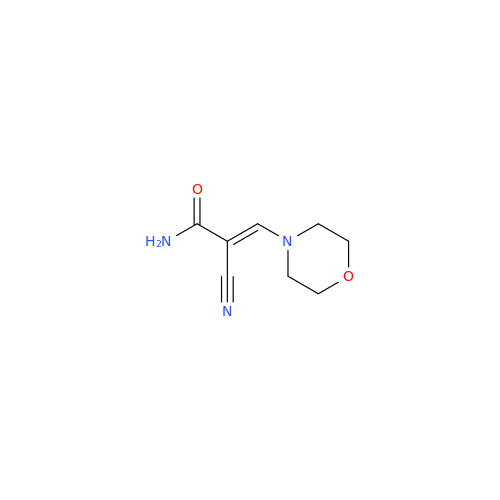
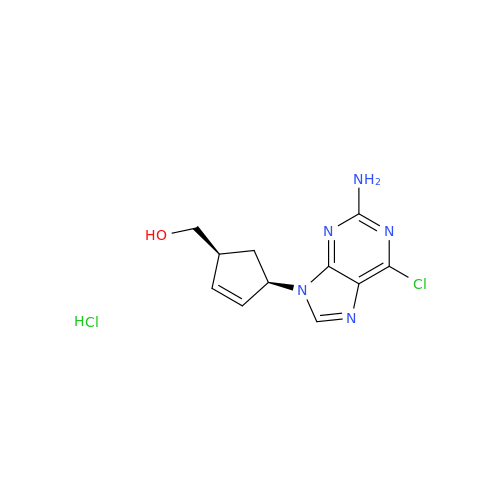
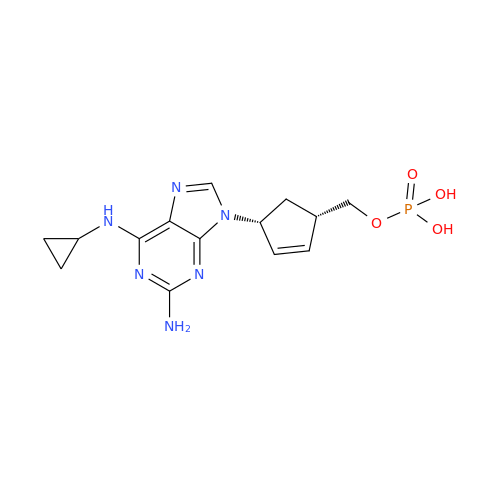
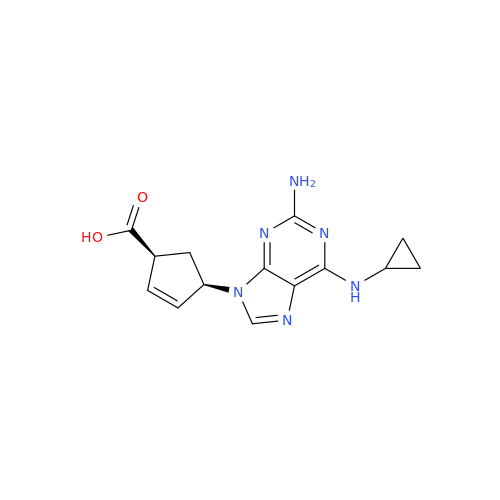
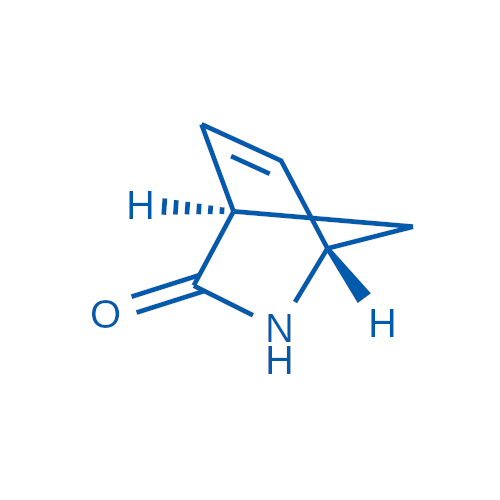
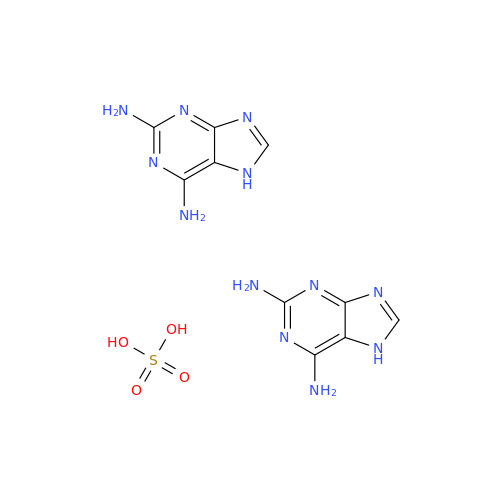
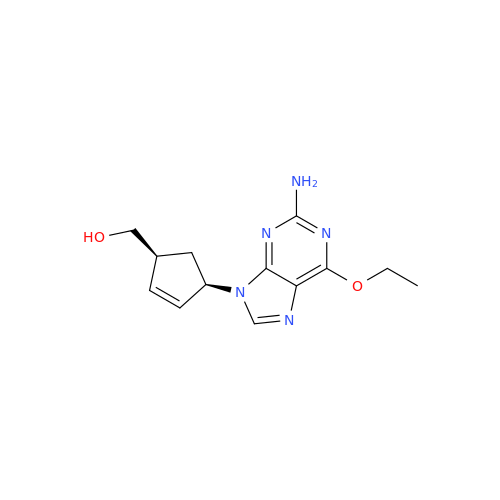
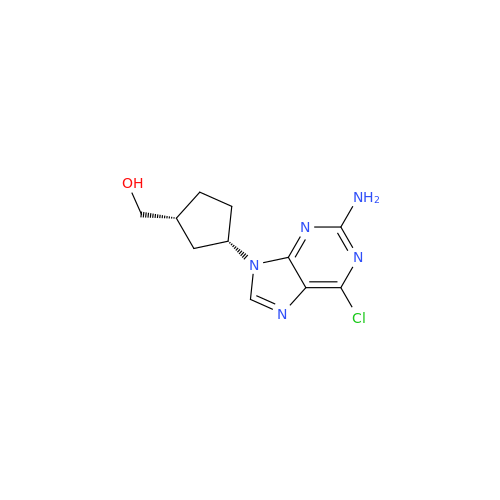
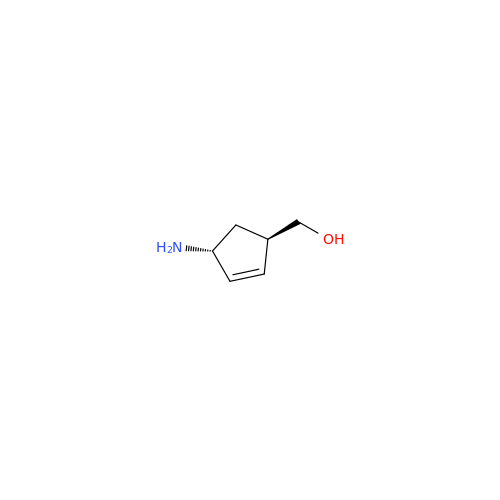
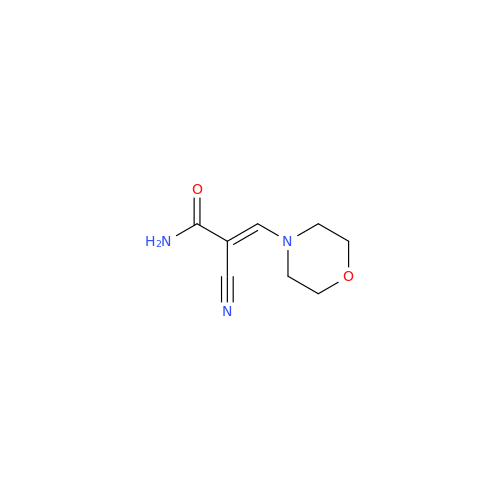
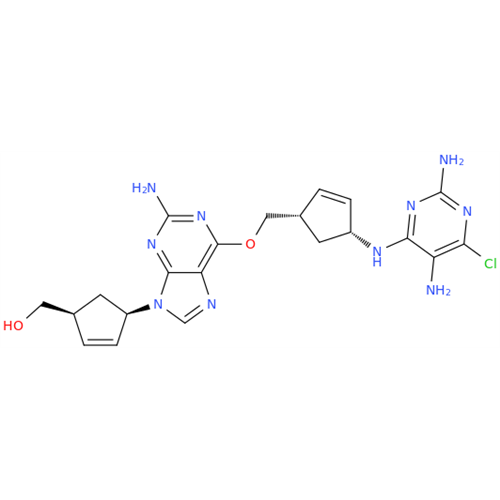
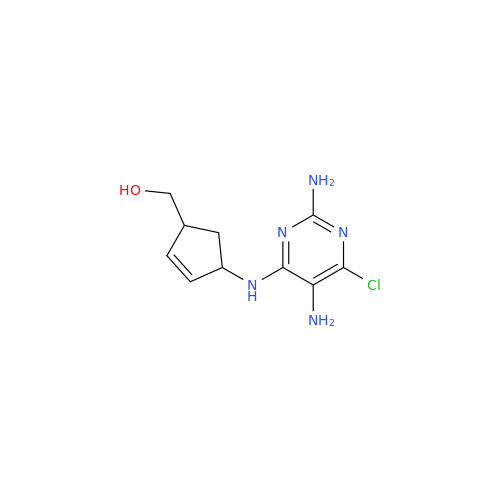
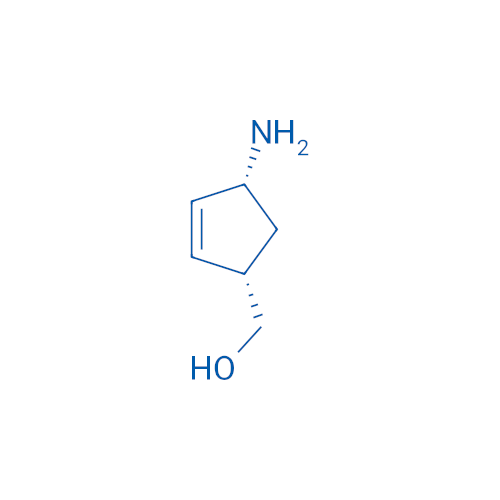
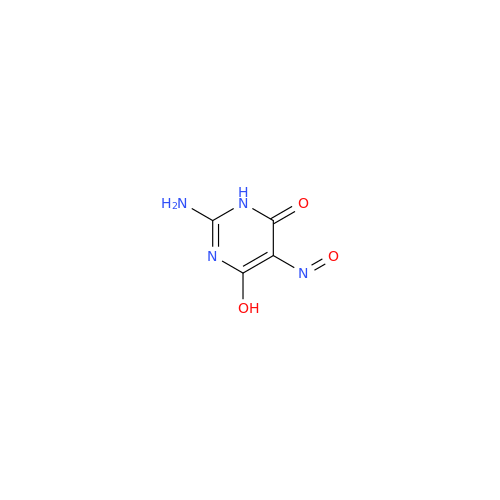
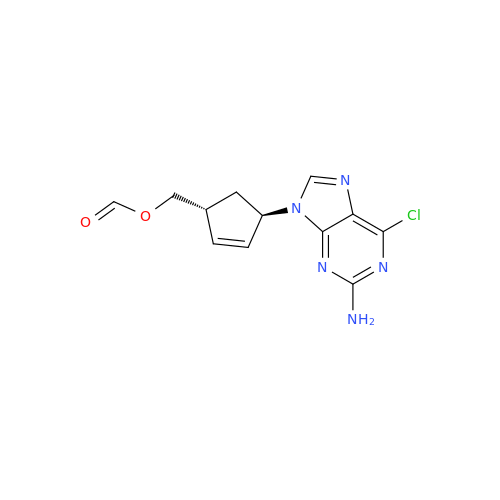
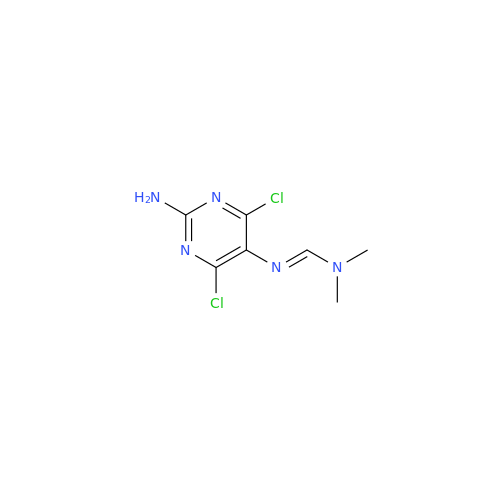
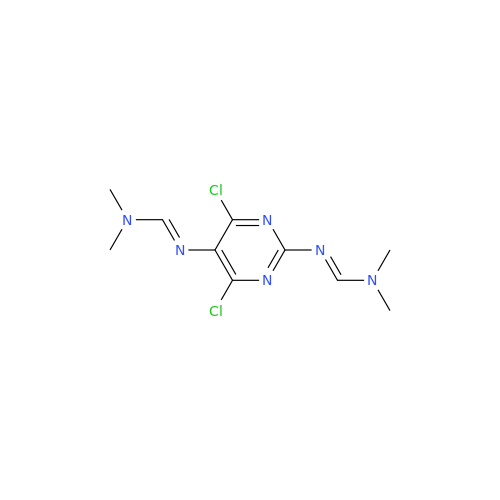
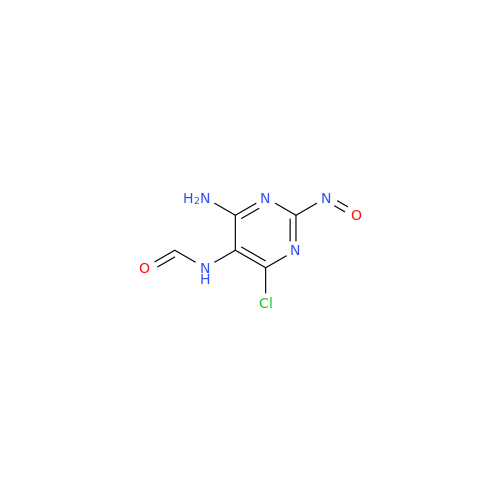
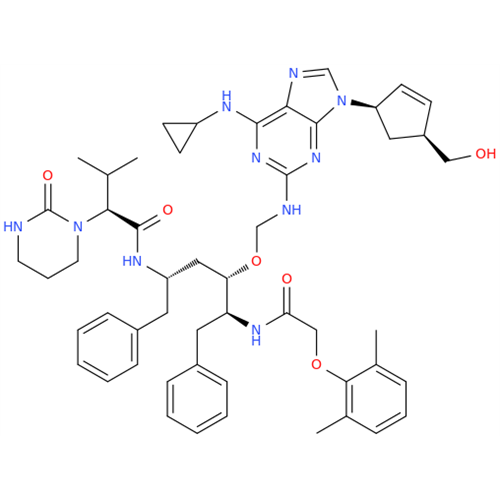
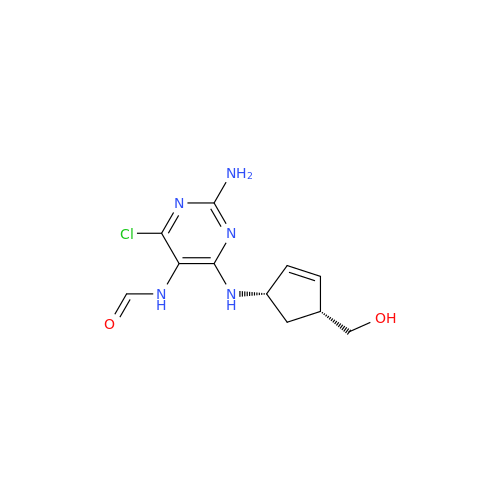
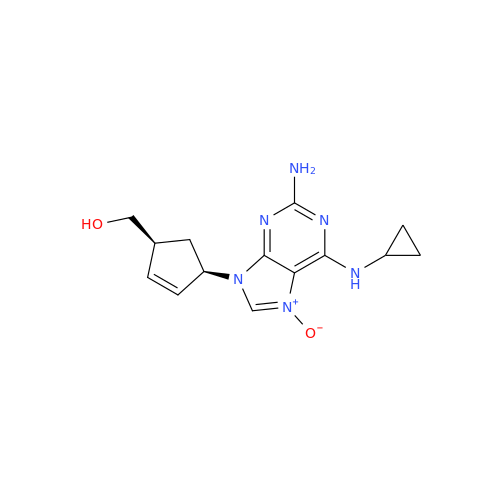
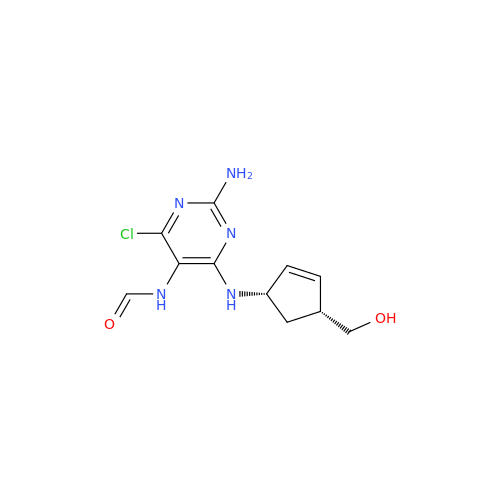
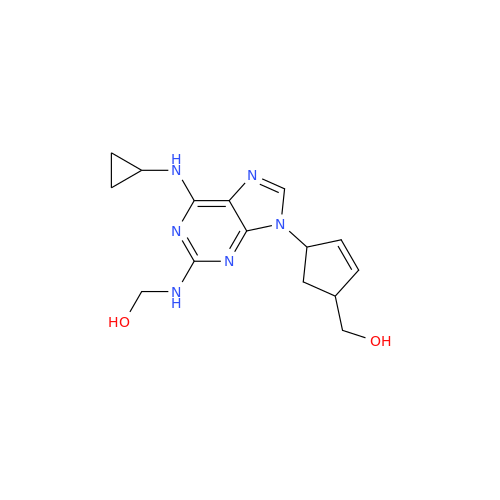
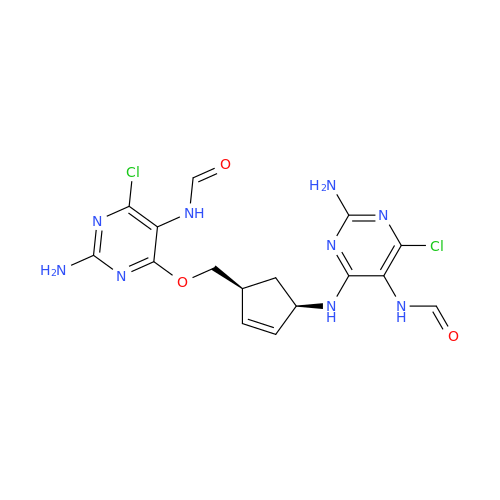
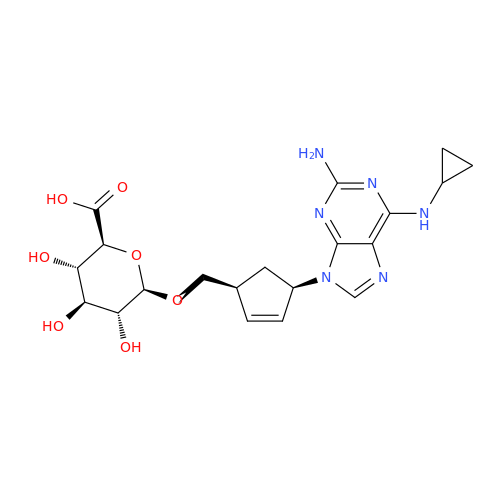
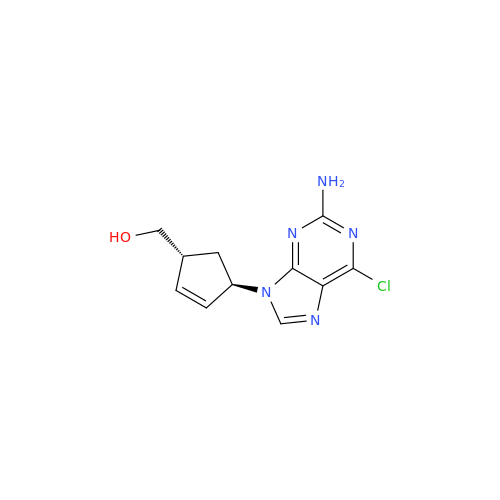
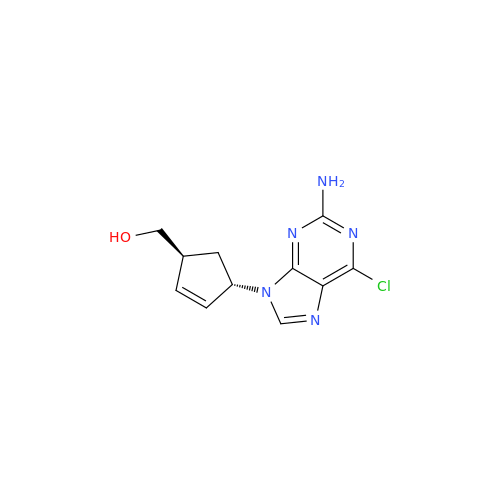
Abacavir Methanolate Impurity
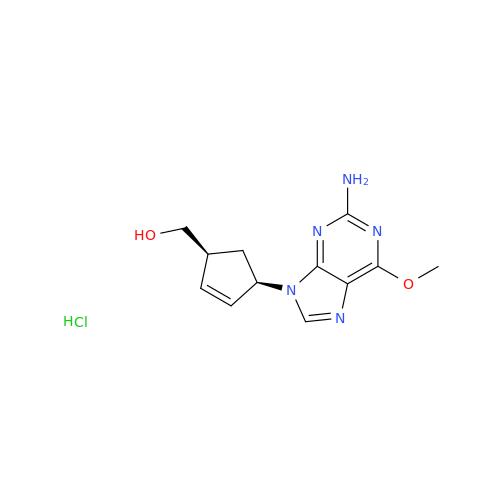
|
Chemical Name: Abacavir Methanolate Impurity
Synonym: Abacavir Methanol Adduct; Abacavir MeOH Clathrate| Enter Batch Number | |||