Product Information
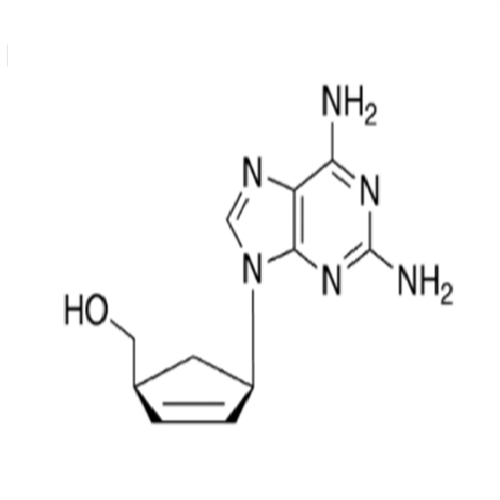
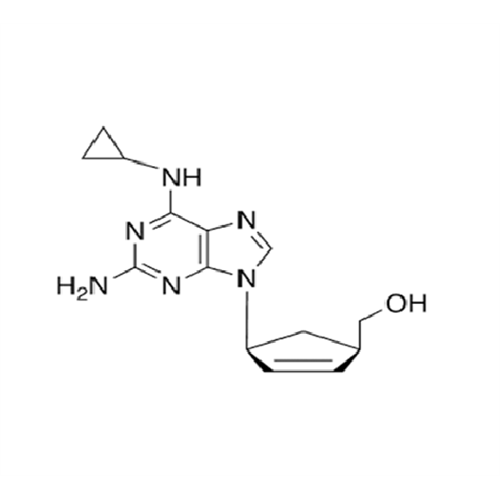
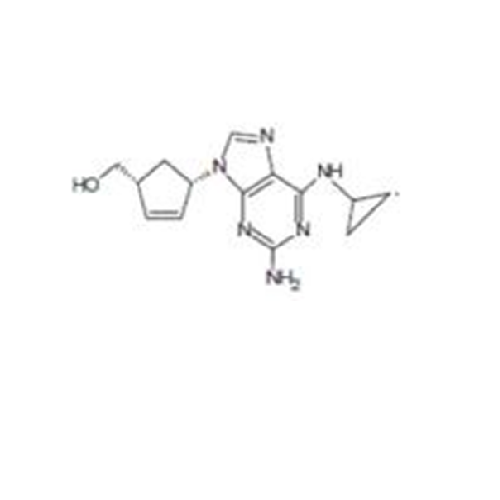
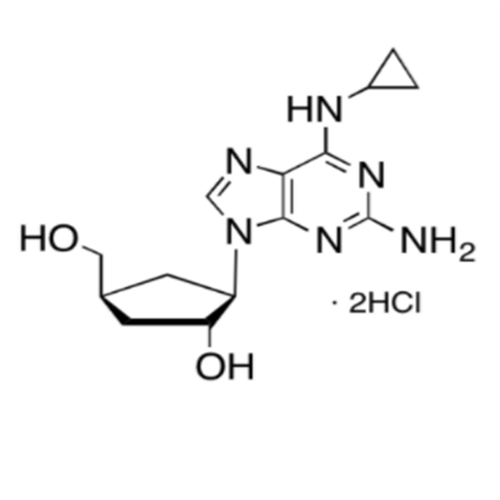
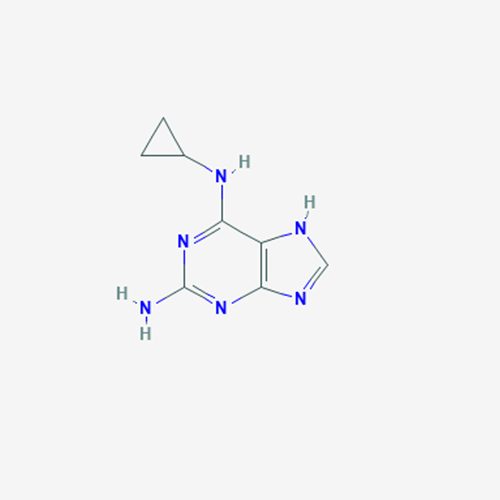
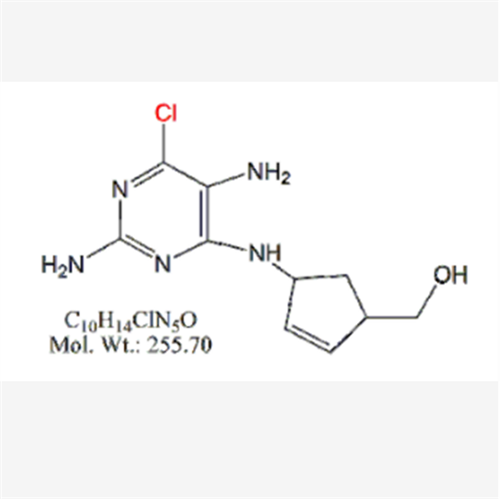
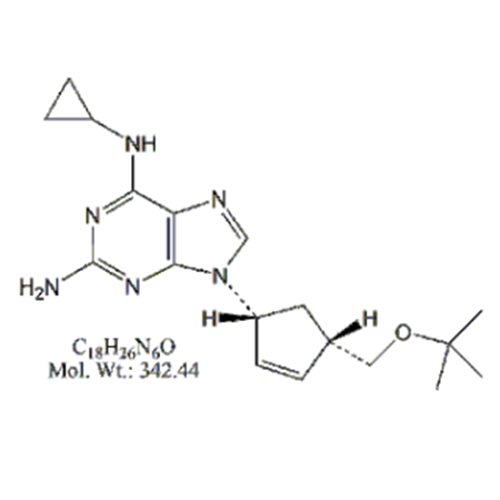
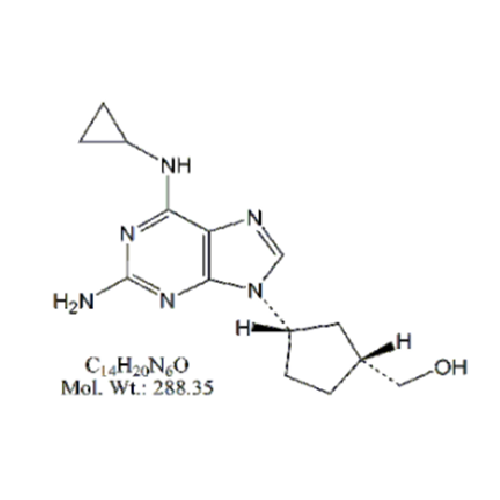
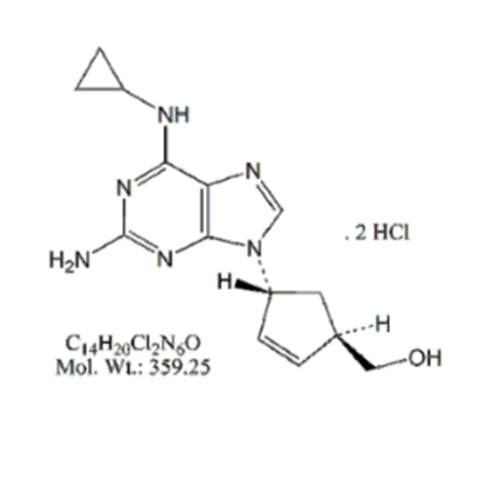
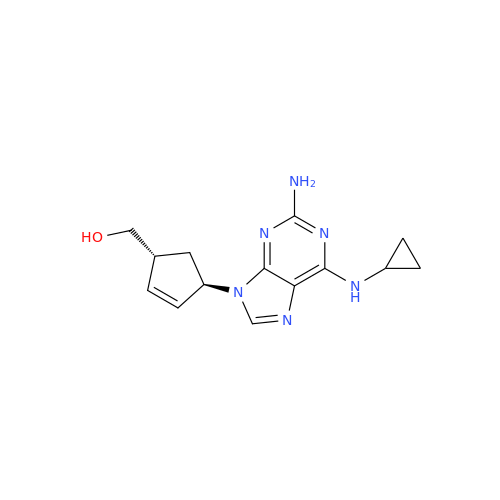
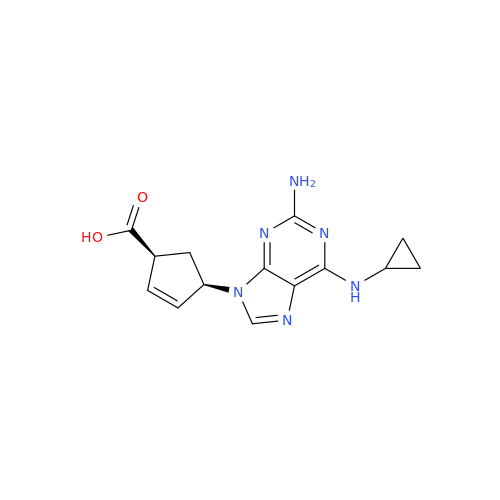
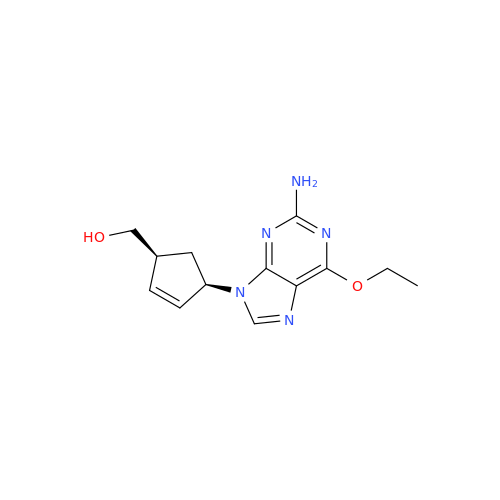
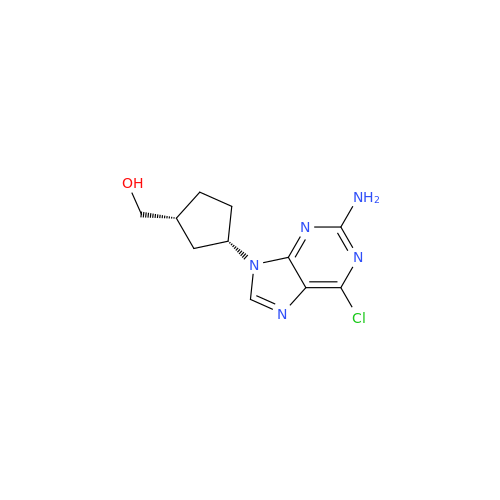
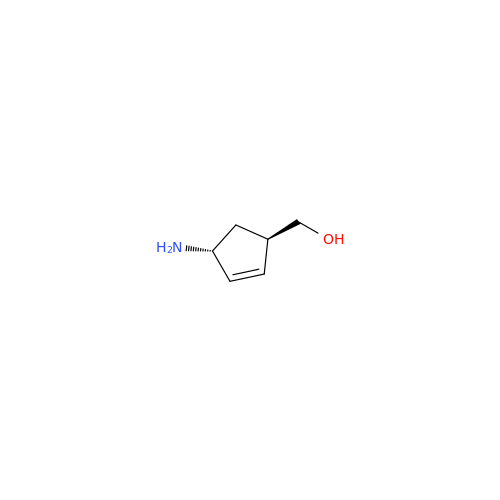
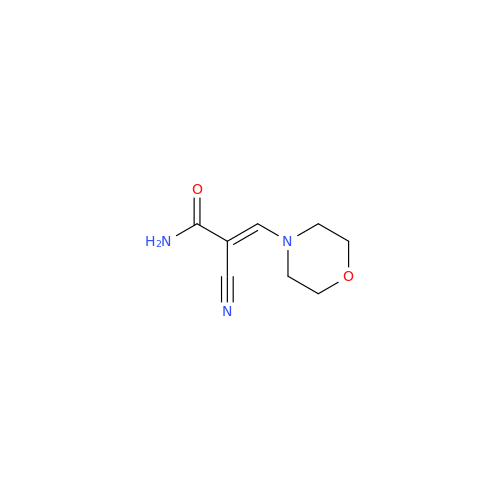
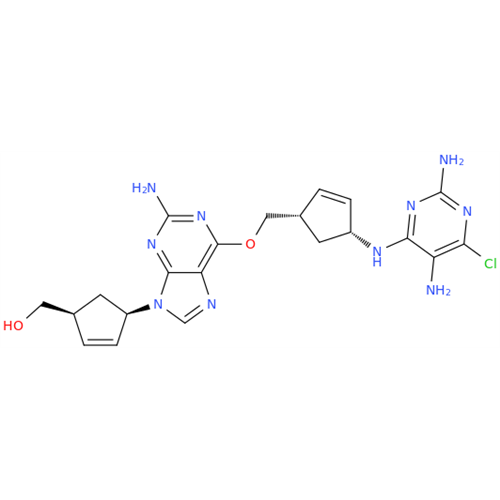
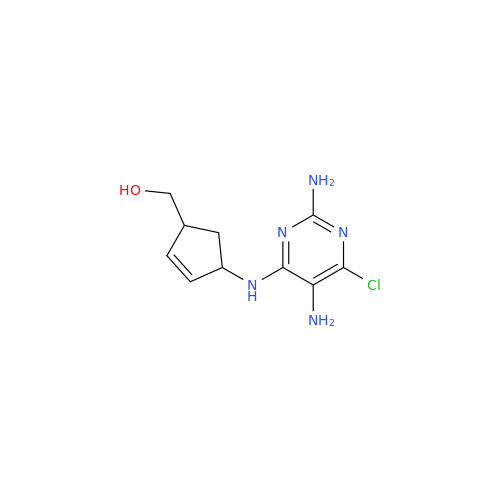
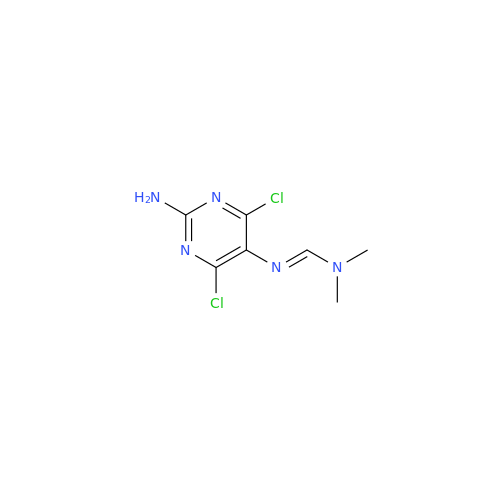
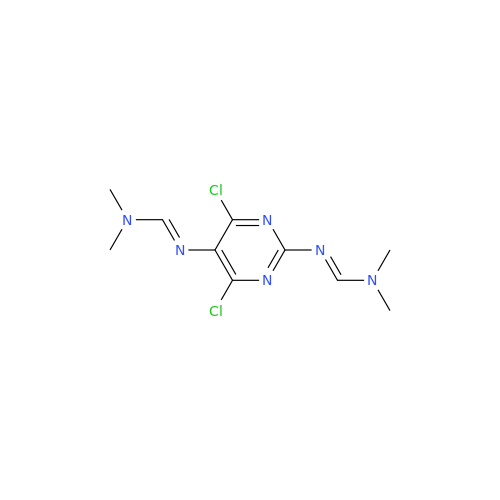
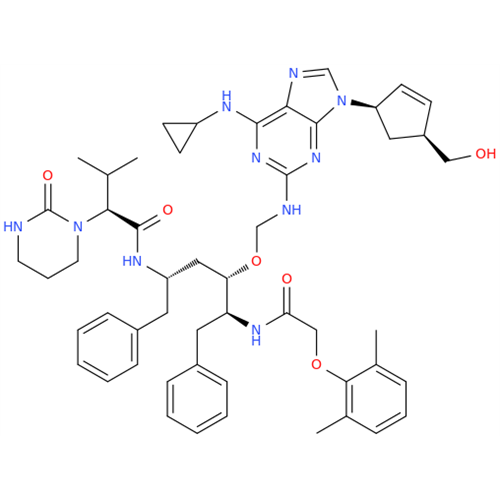
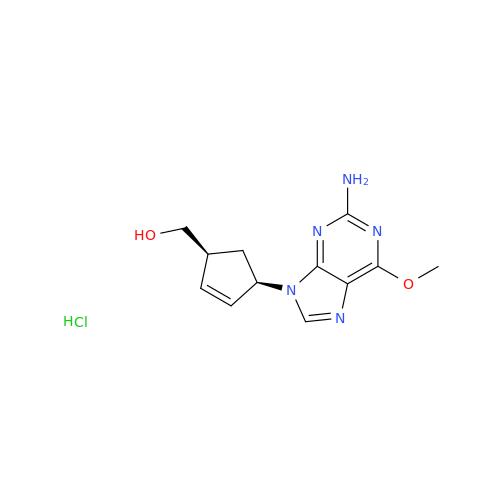
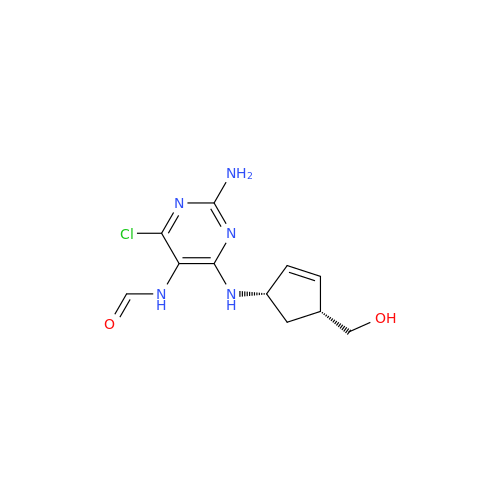
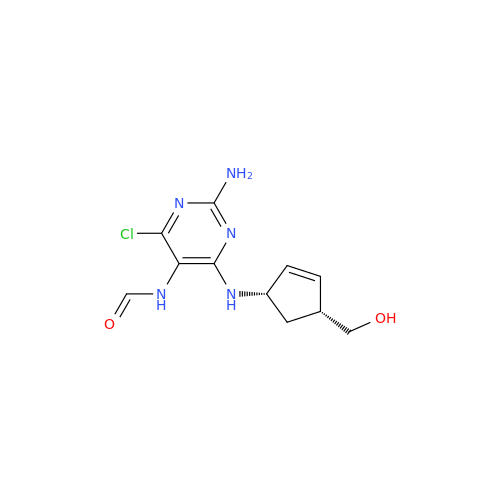
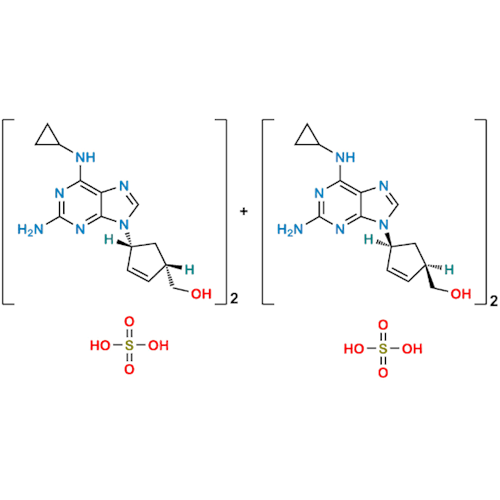
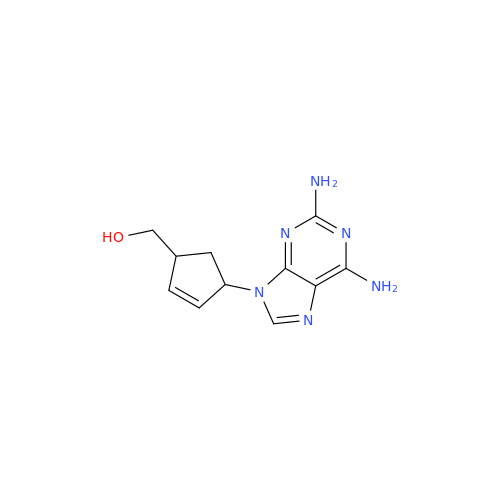
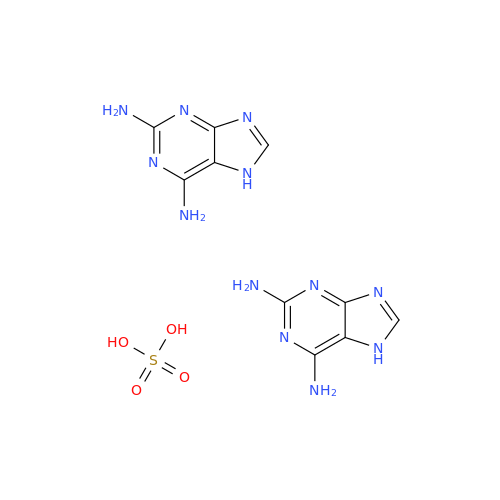
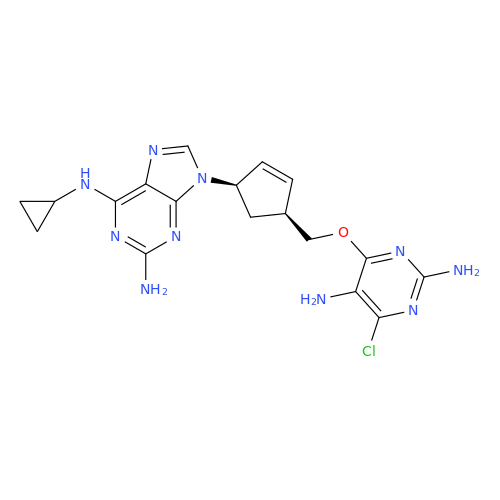
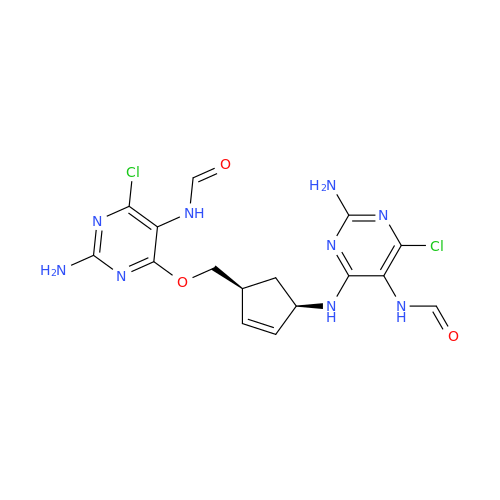
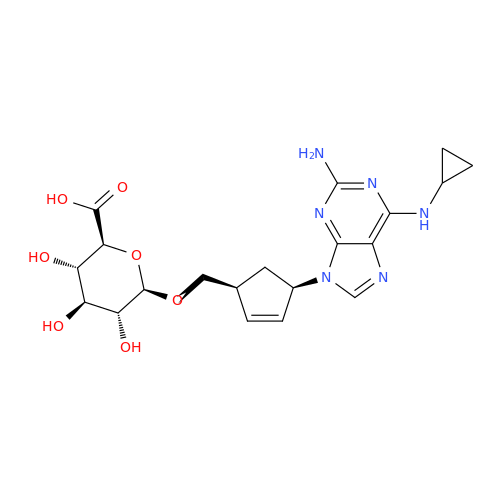
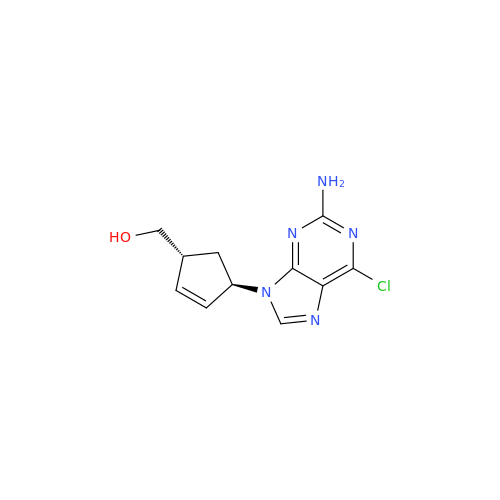
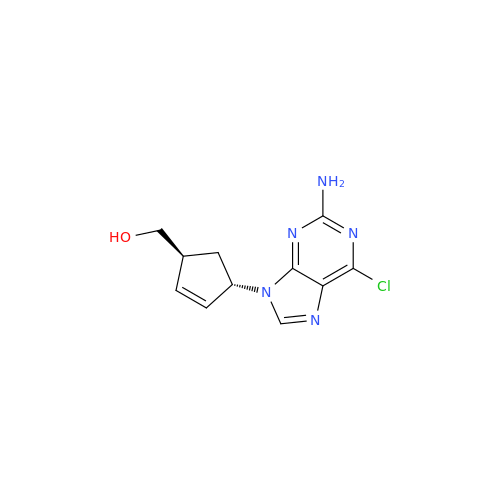
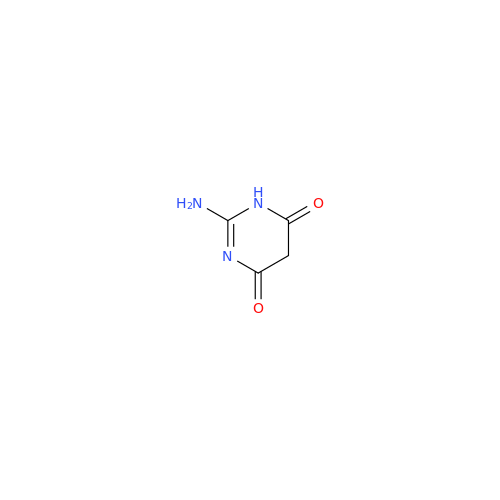
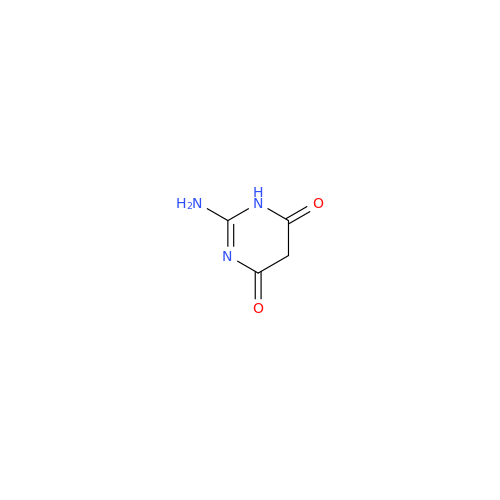
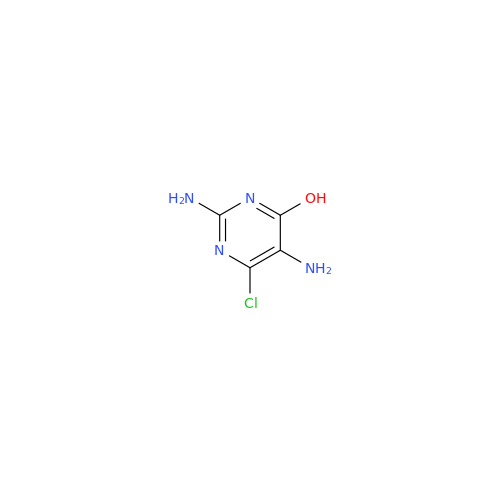
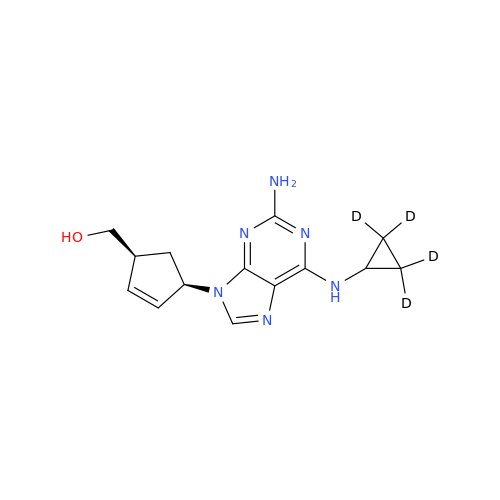
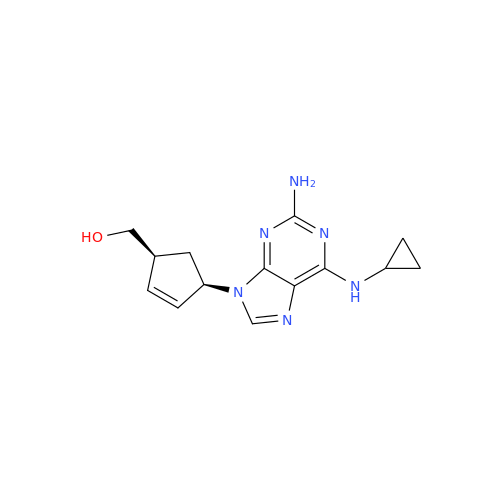
Abacavir Impurity 9
|
Chemical Name: Abacavir Impurity 9
Synonym: Abacavir Ethyl Ester Impurity; EP/USP Abacavir Impurity 9| Enter Batch Number | |||