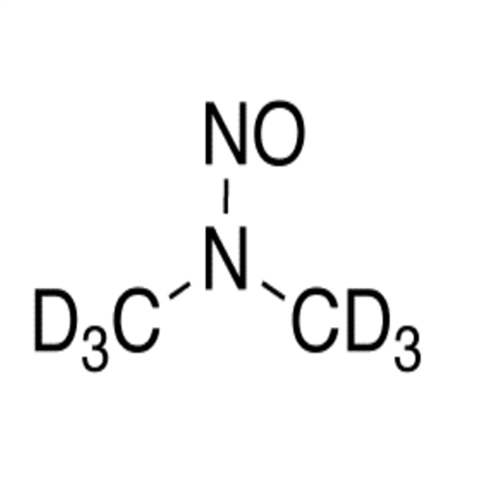
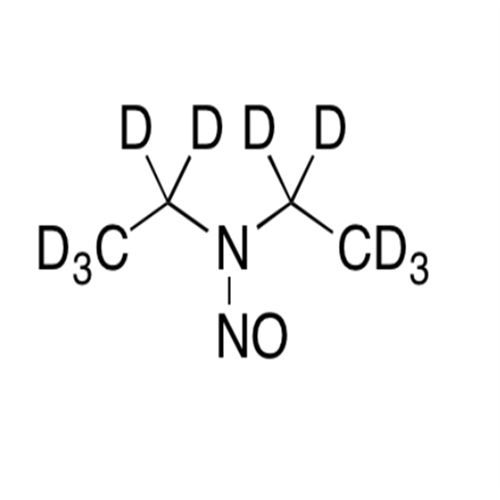
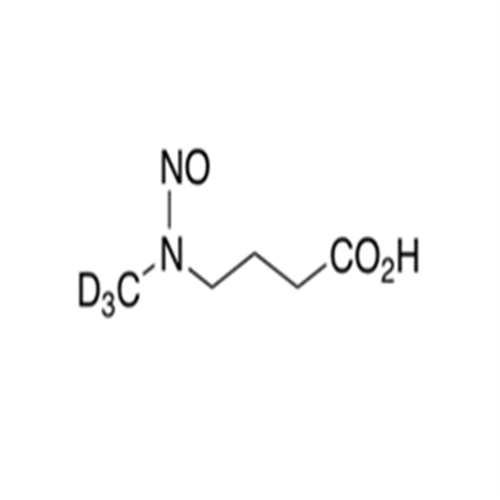
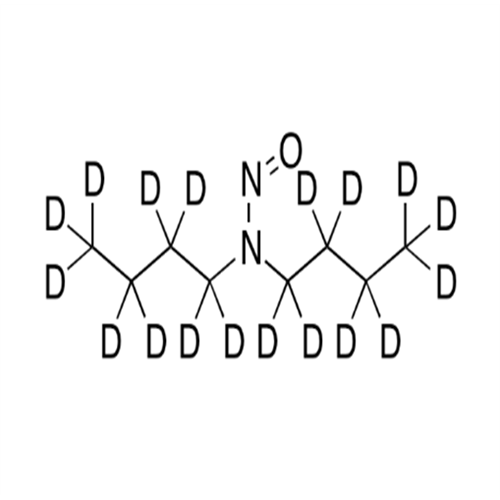
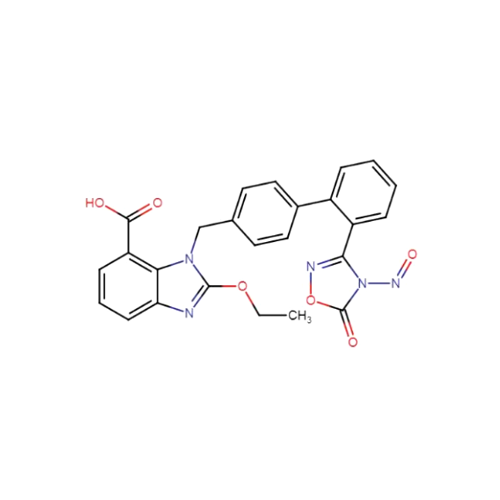
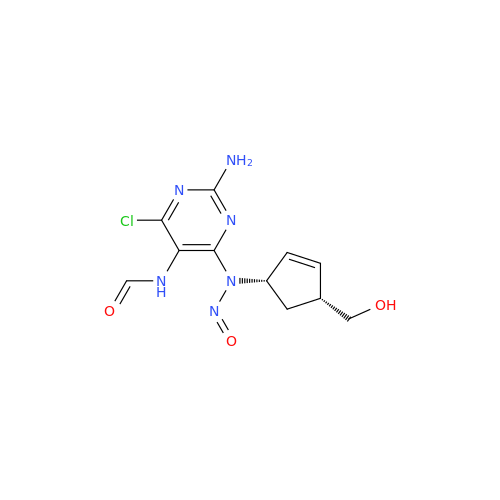
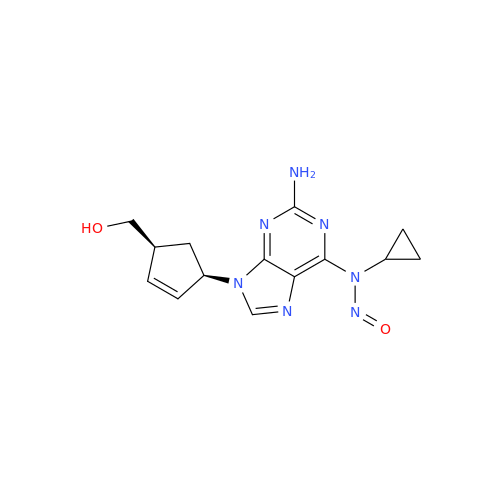
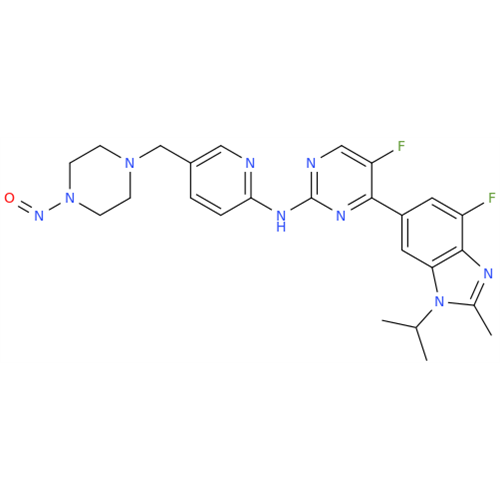
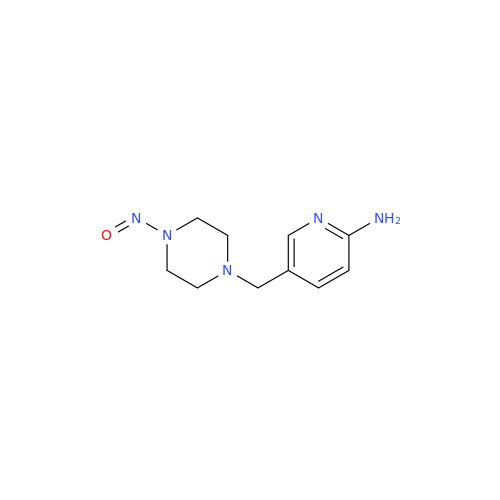
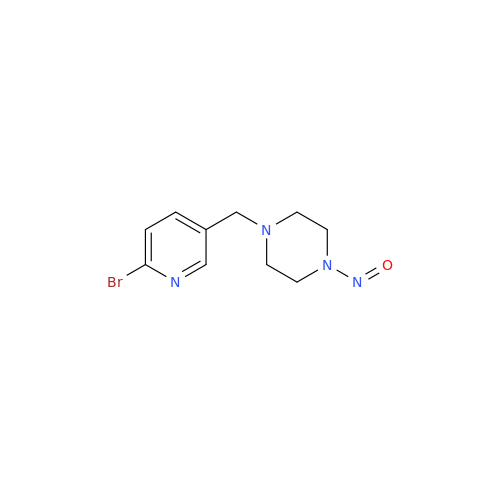
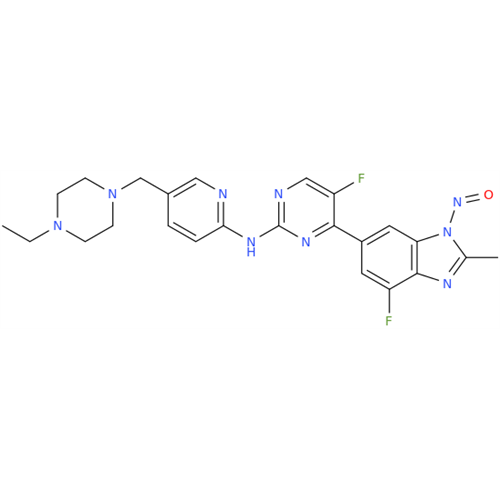
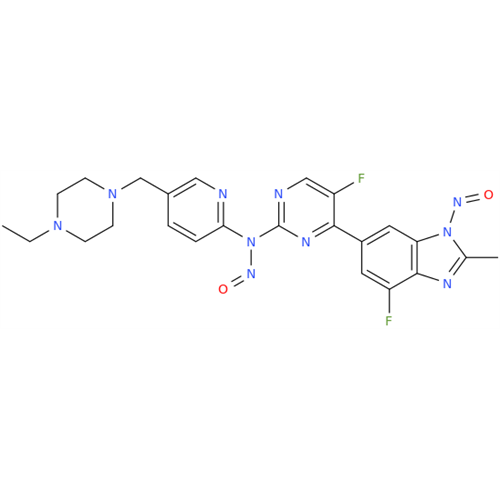
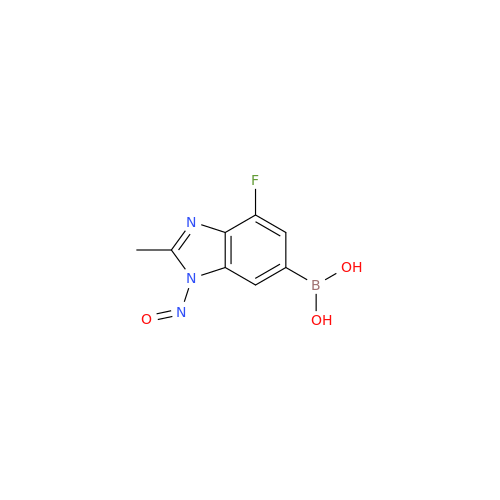
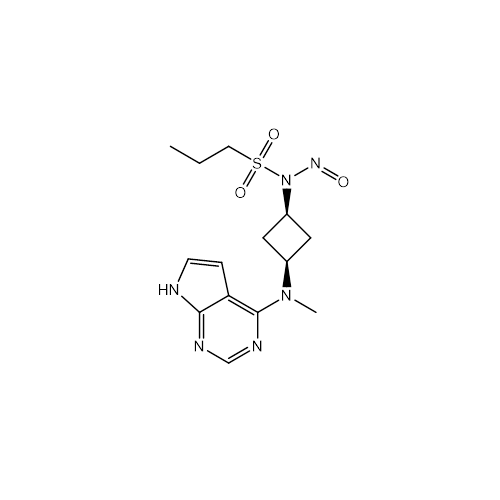
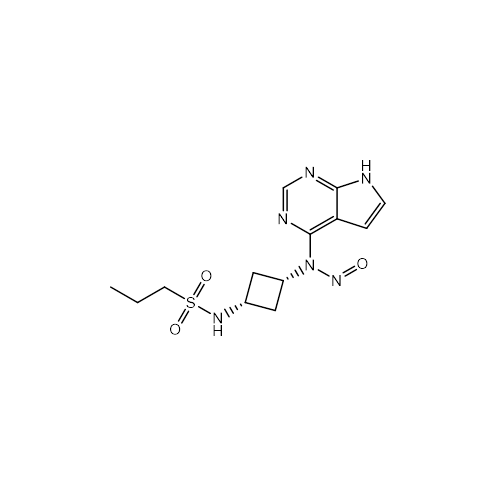
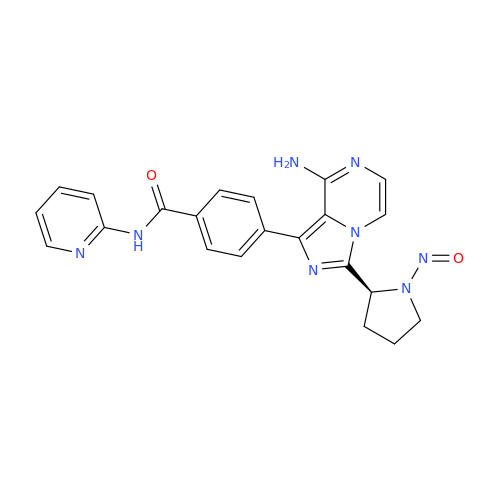
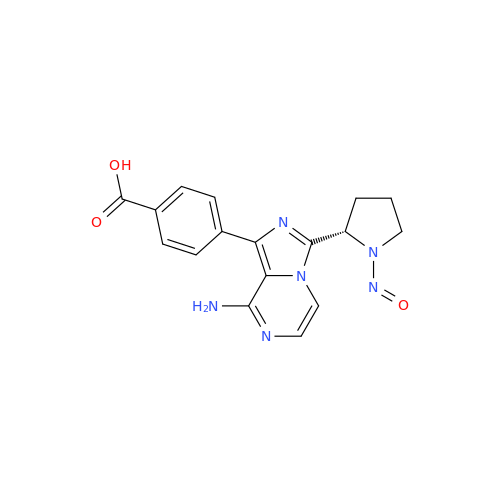
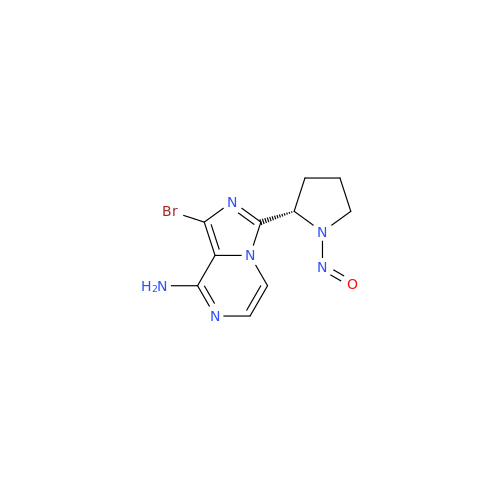
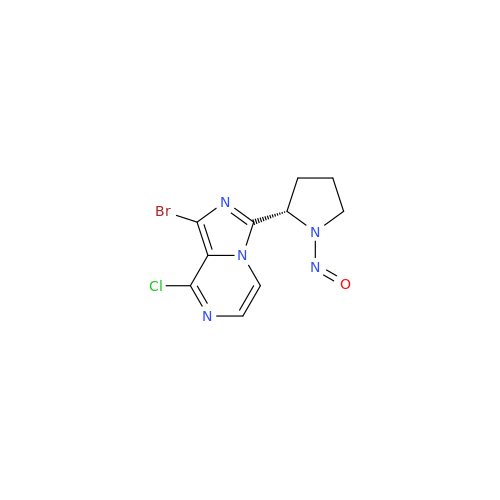
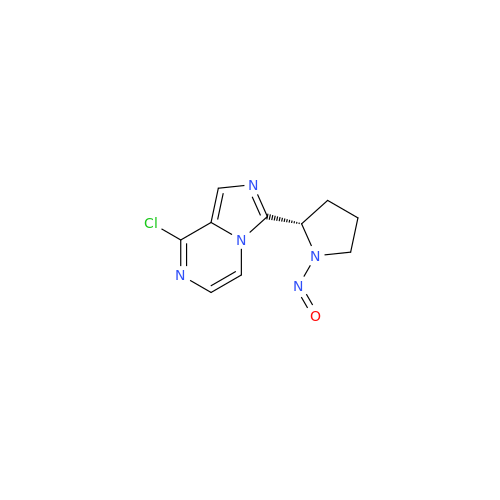
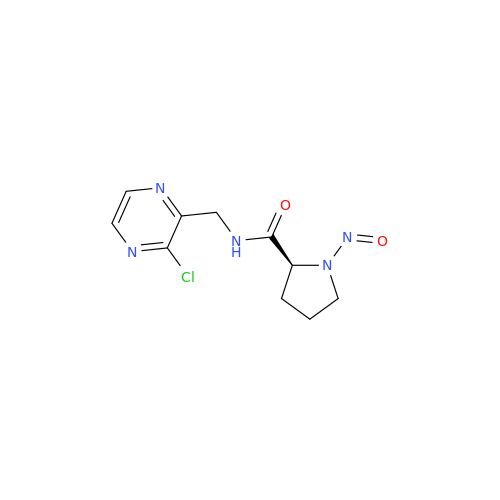
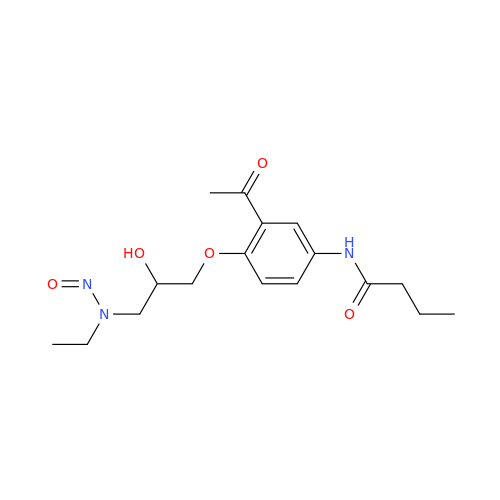
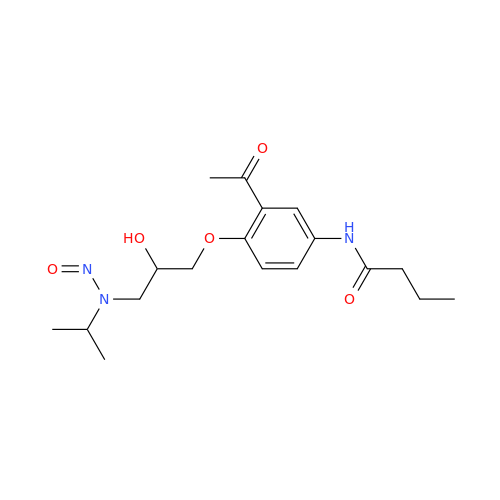
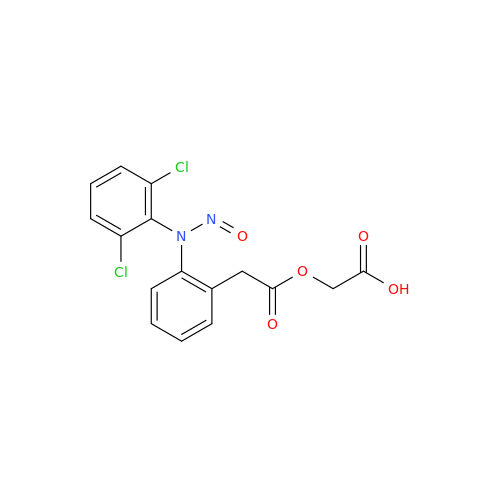
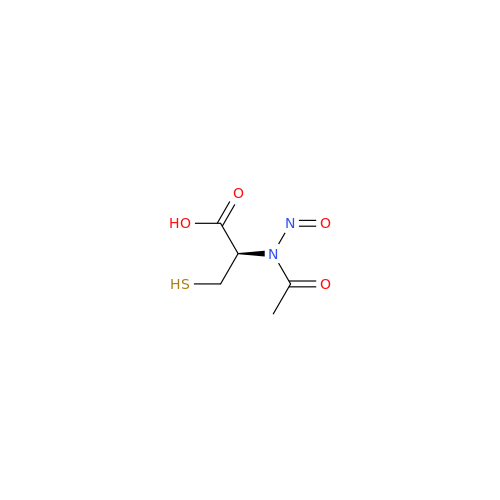
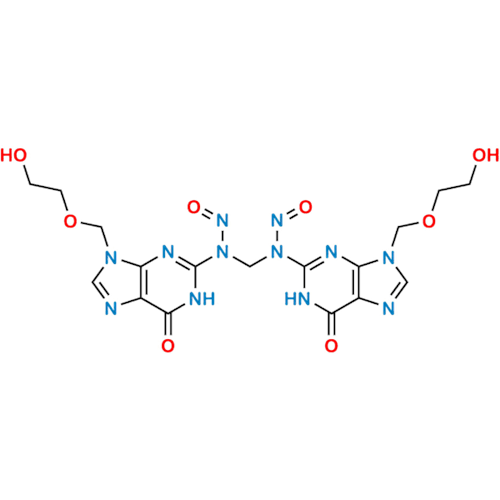
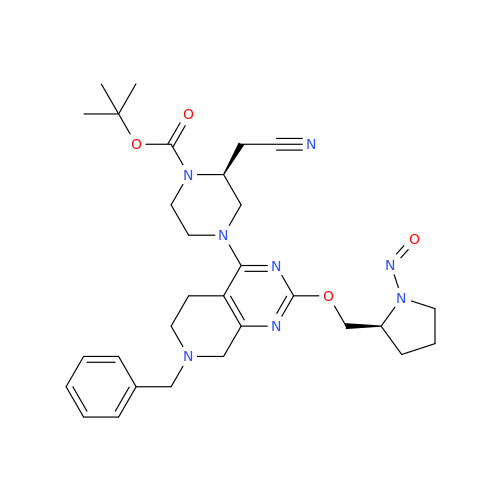
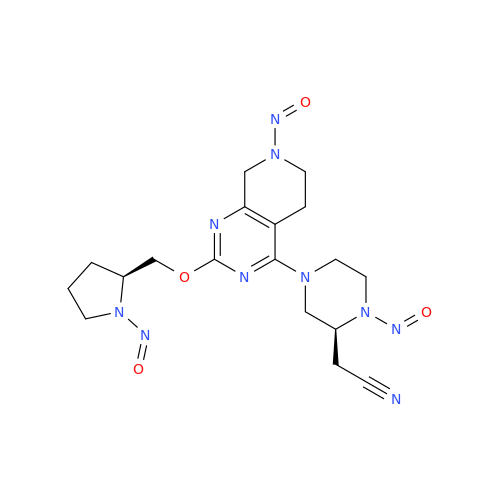
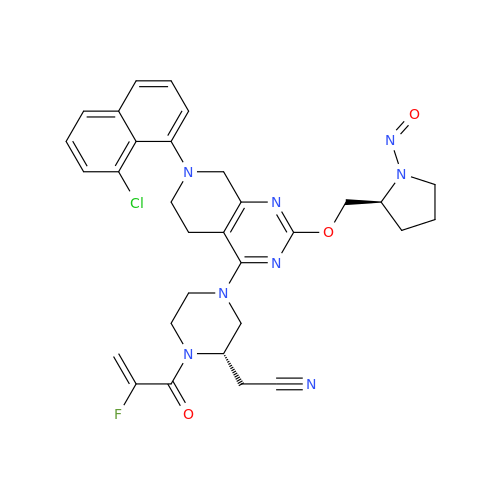
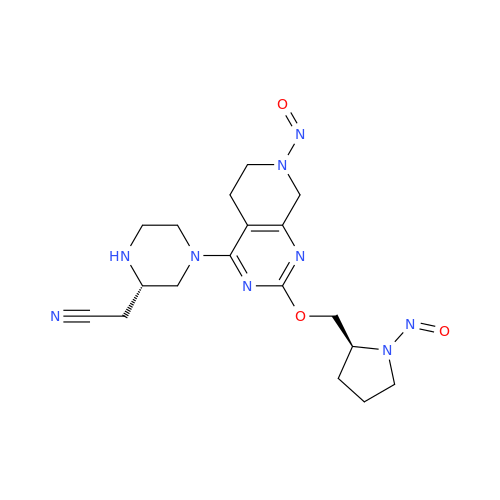
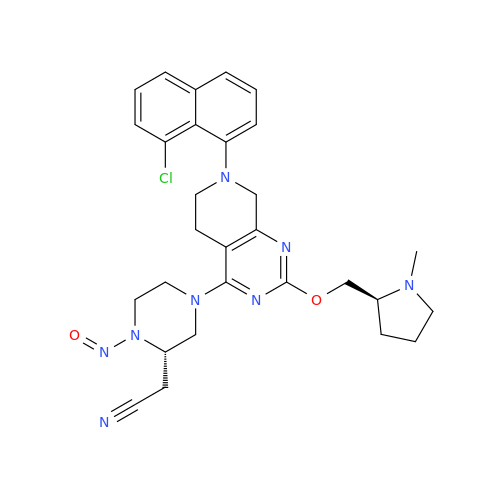
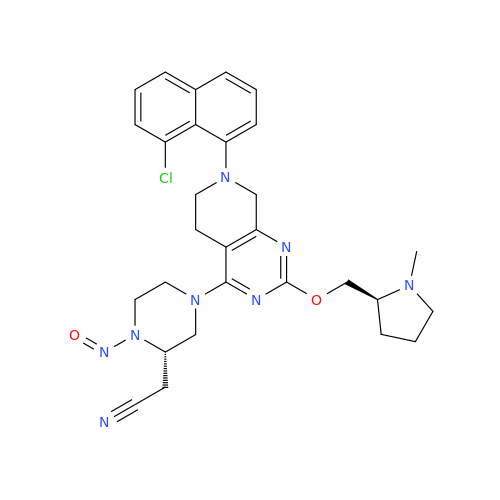
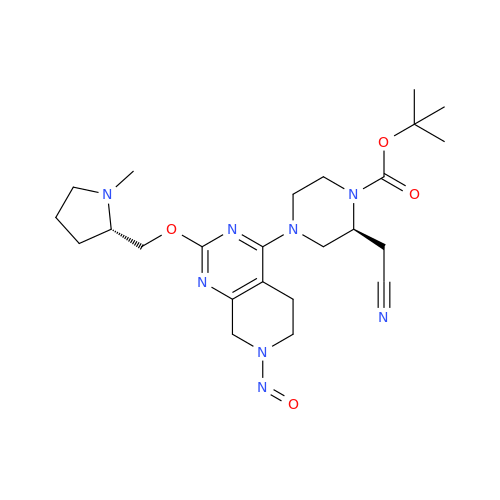
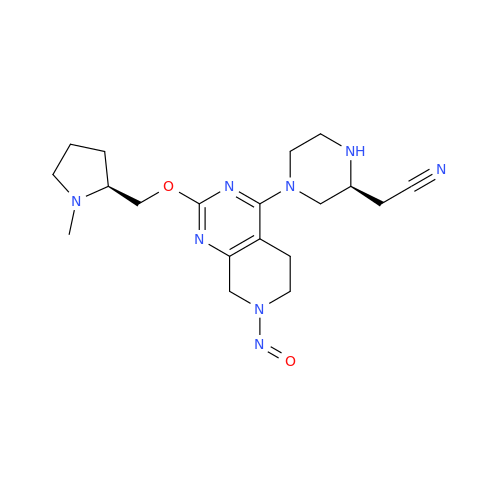
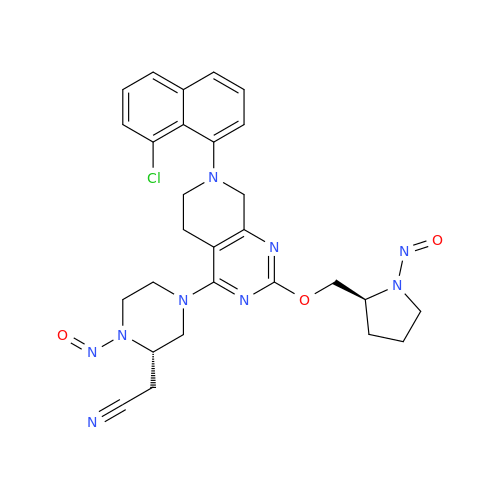
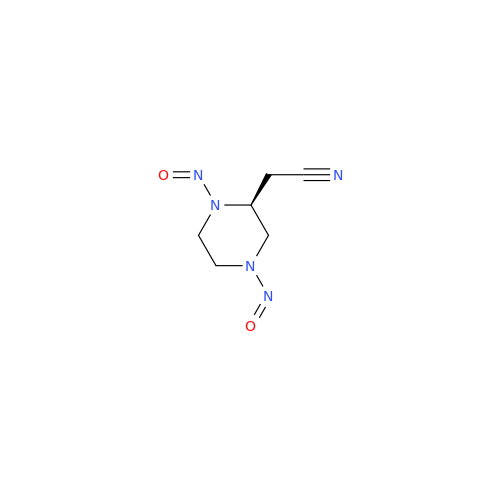
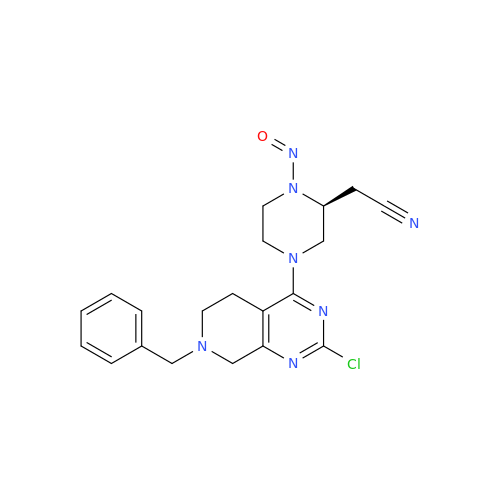
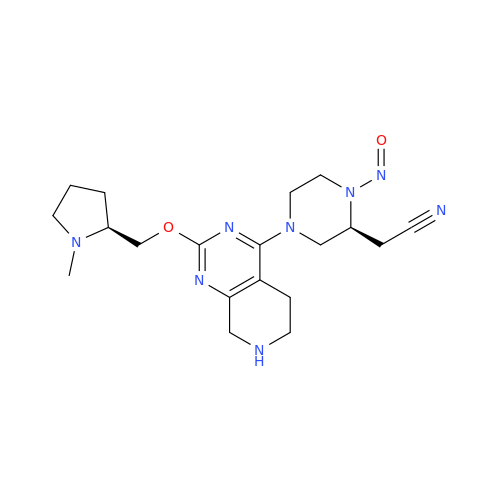
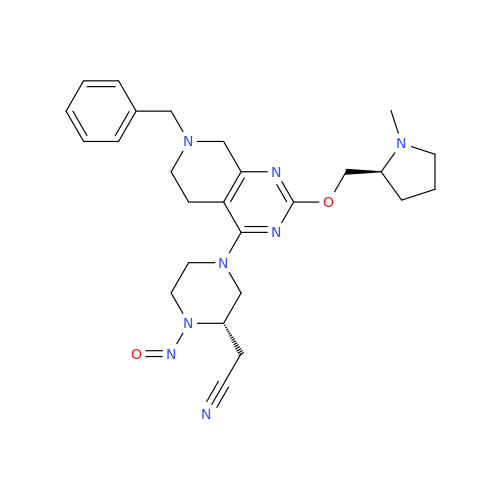
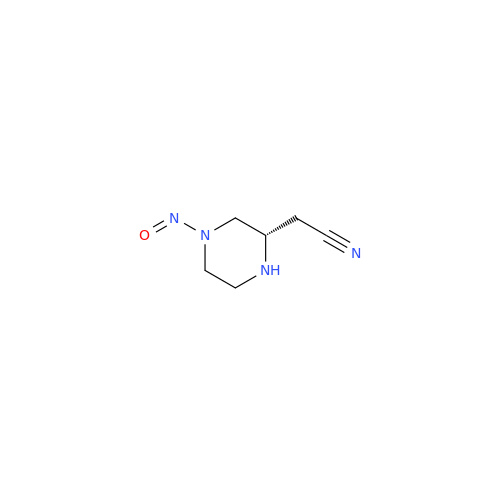
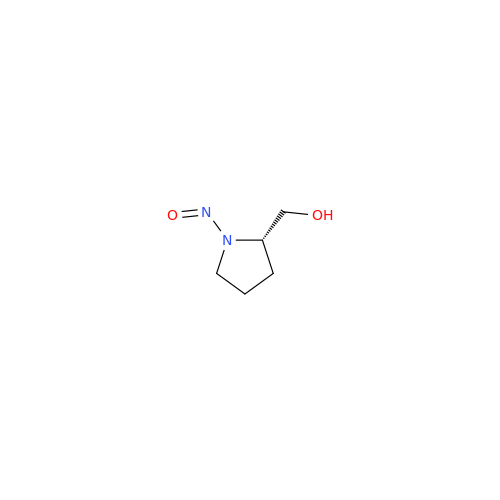
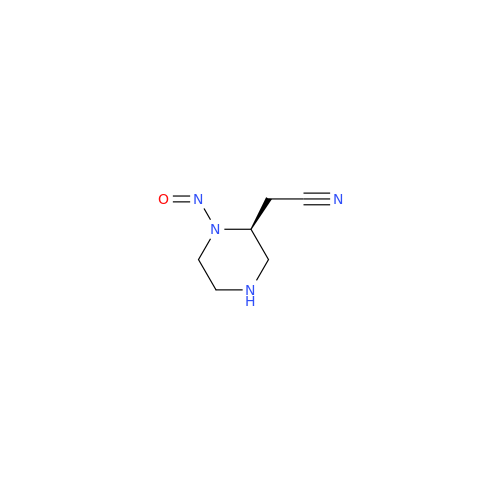
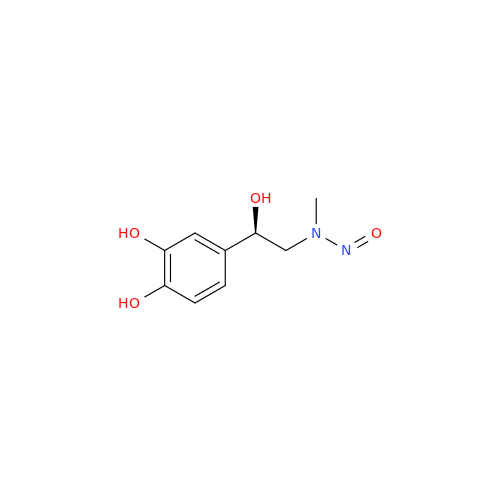
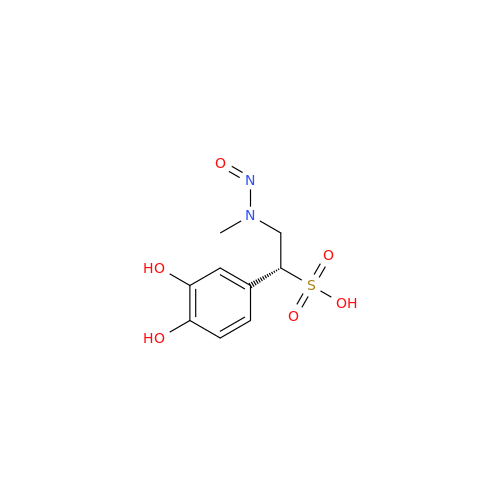
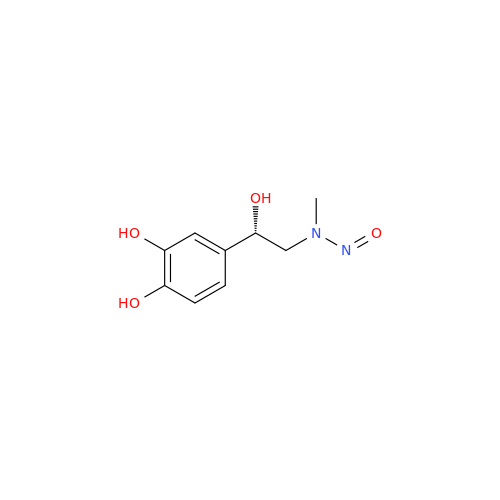
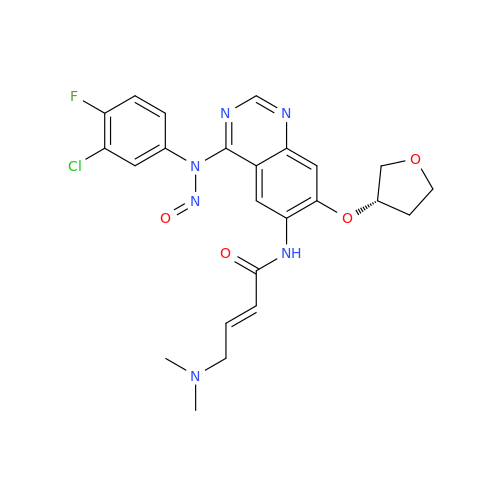
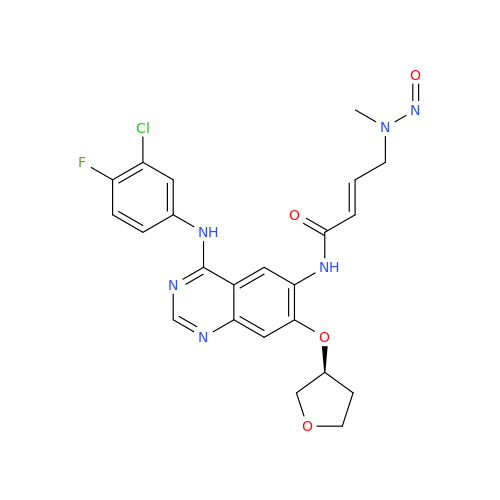
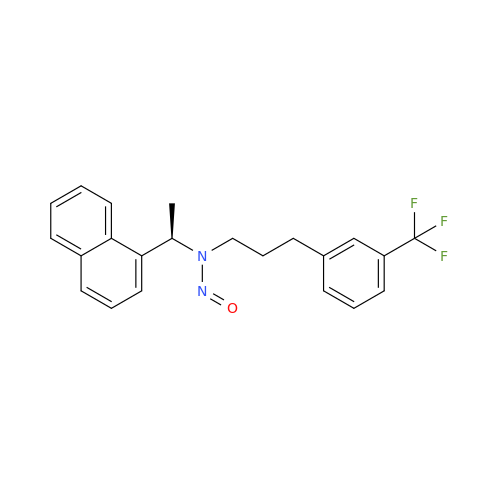
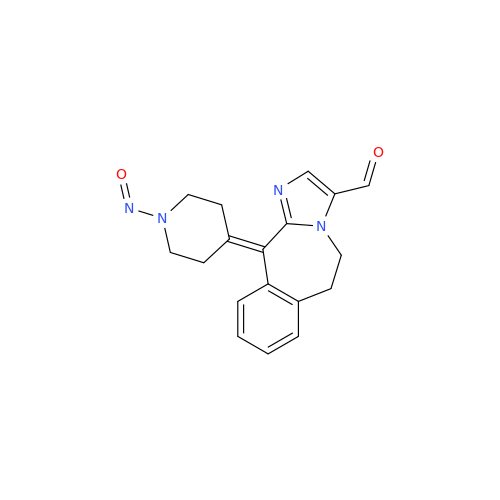
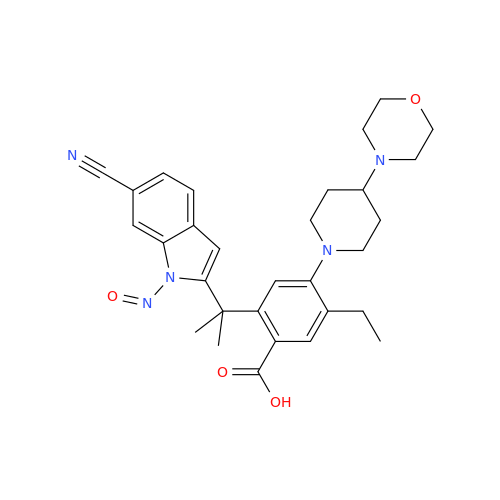
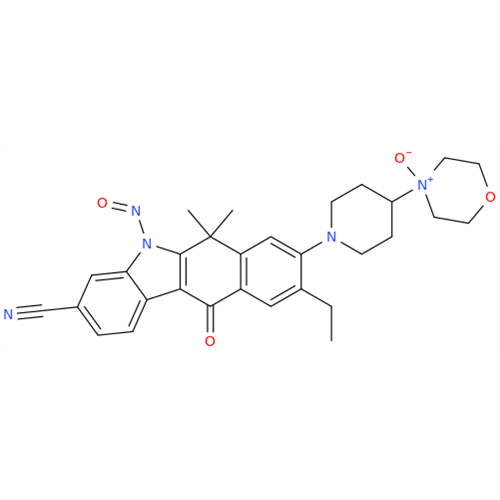
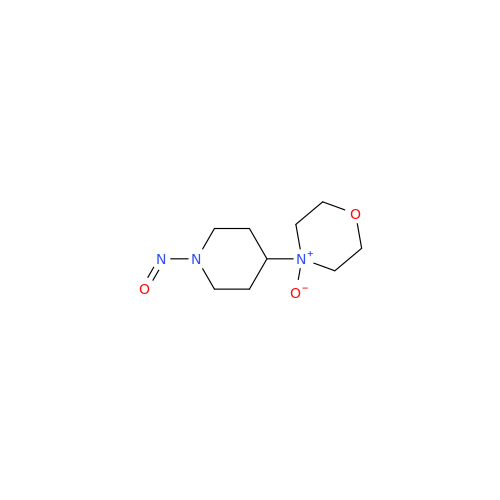
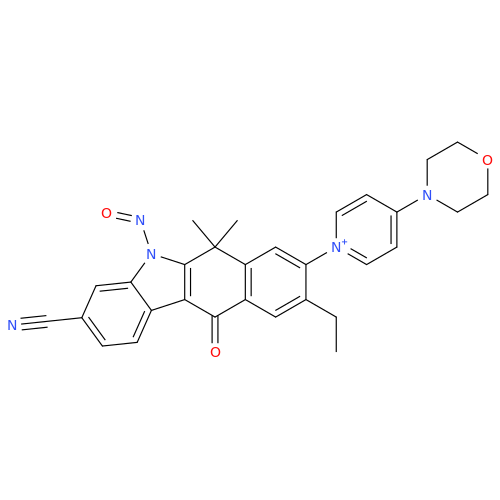
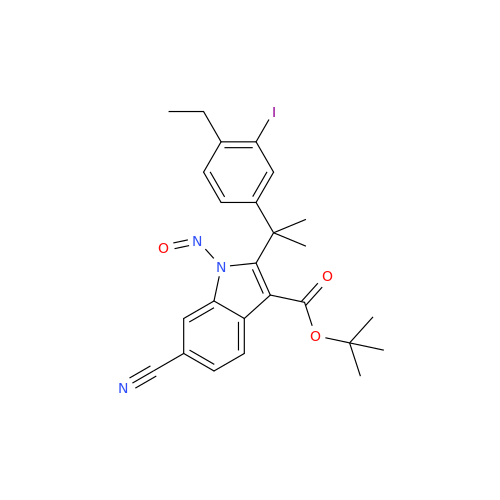
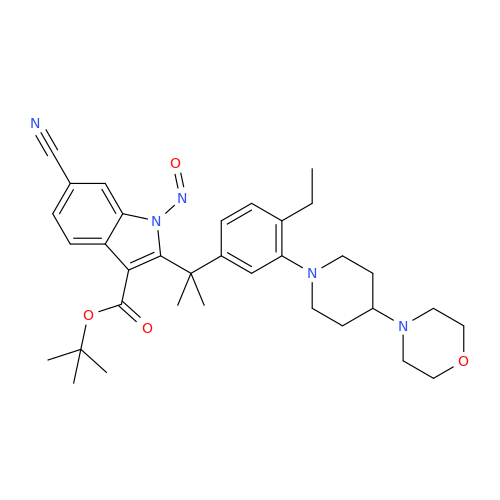
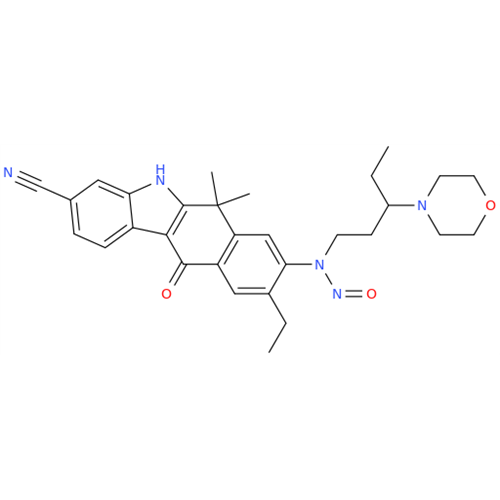
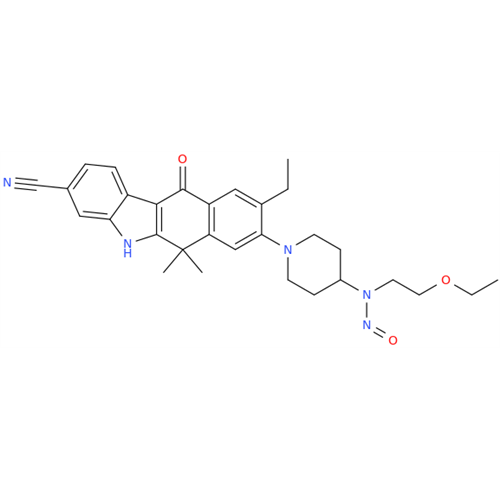
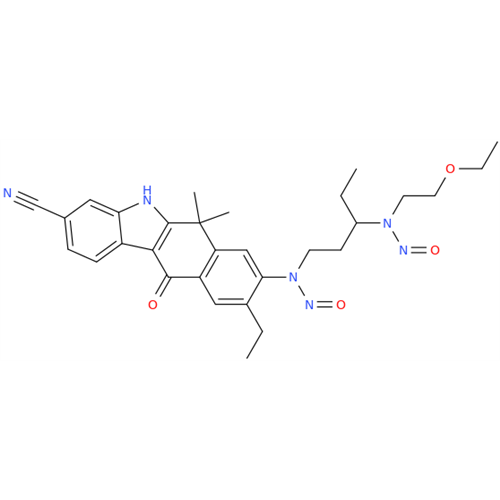
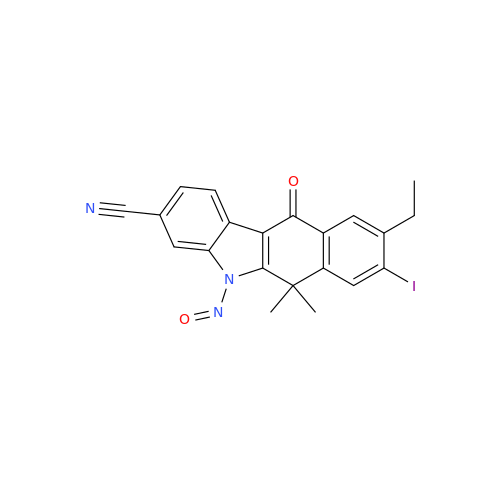
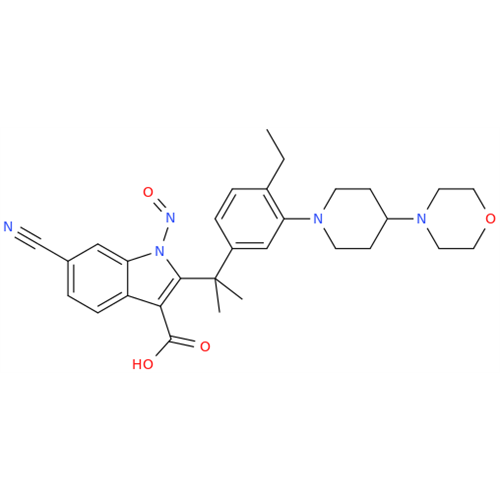
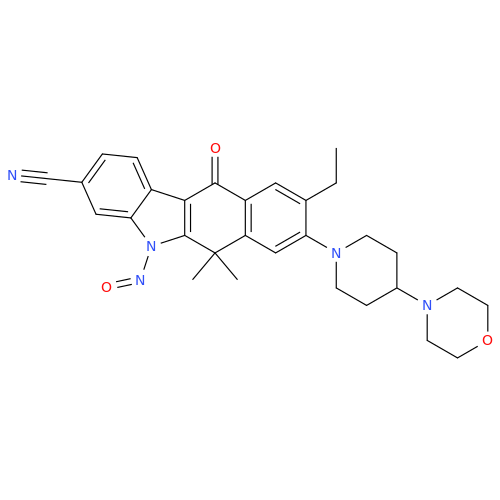
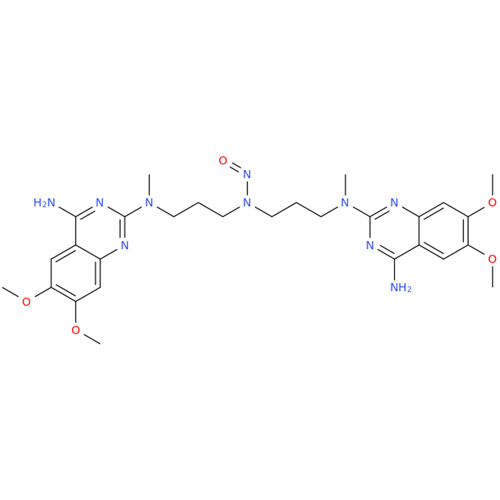
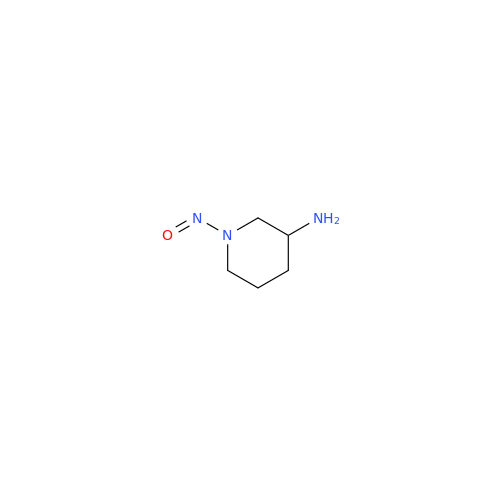
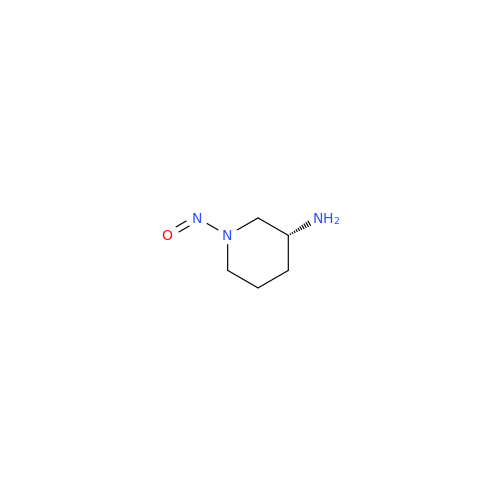
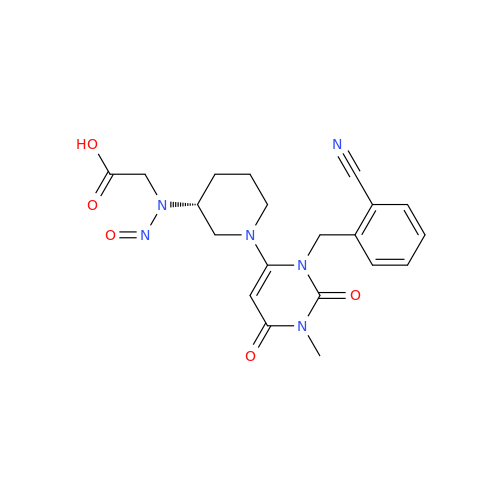
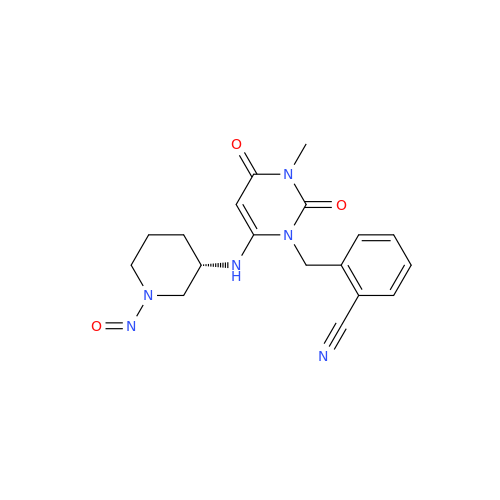
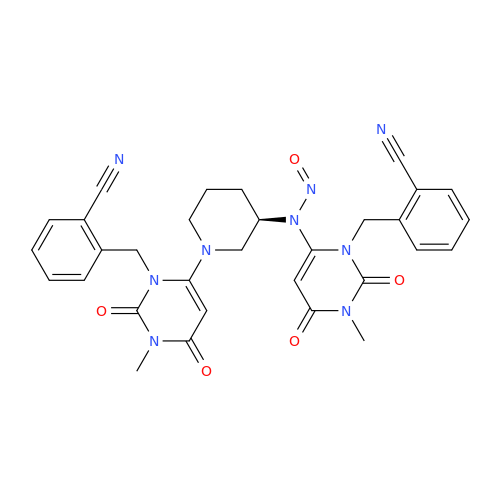
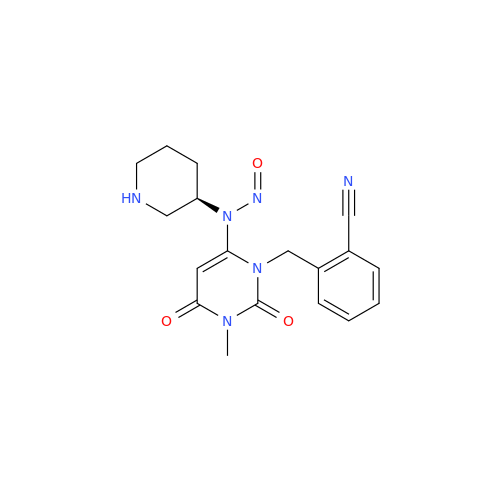
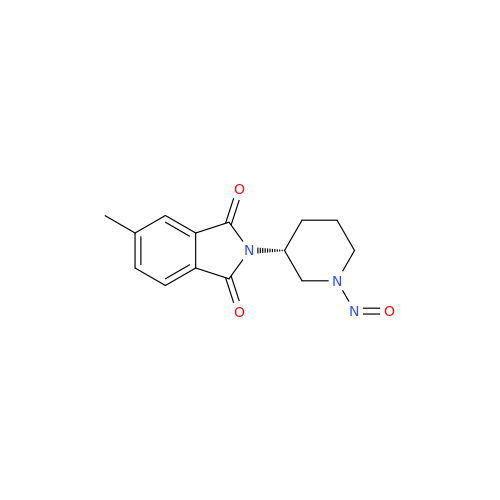
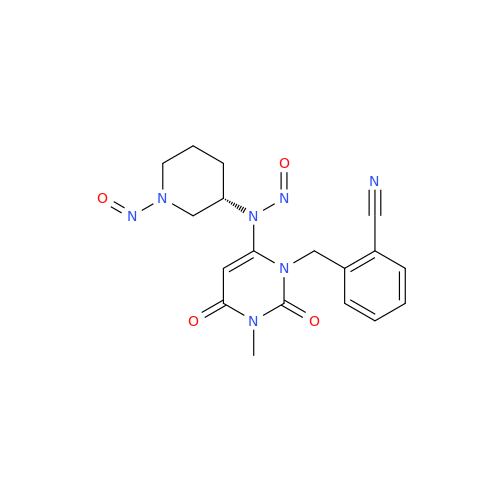
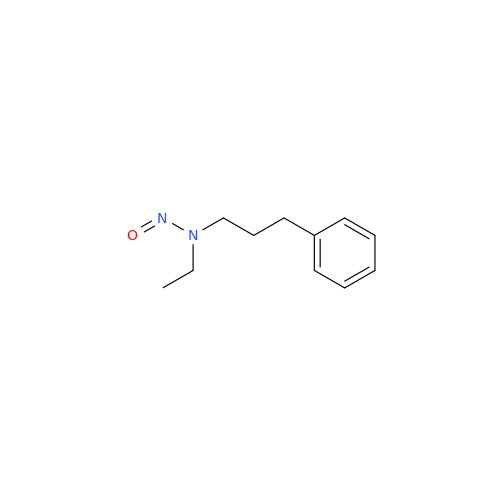
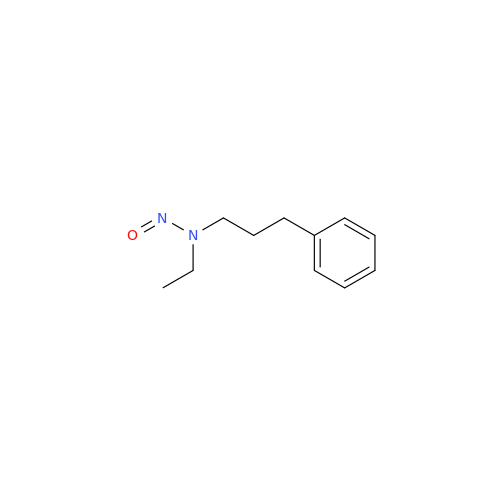
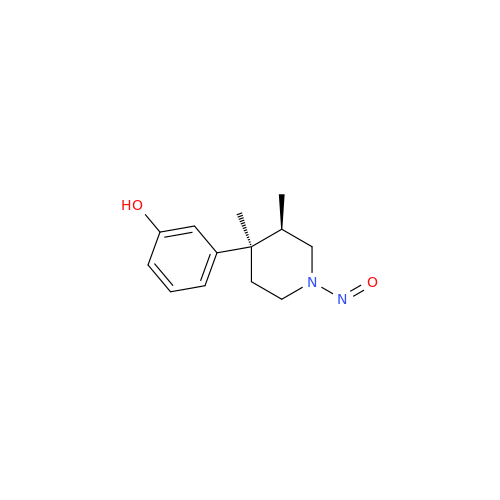
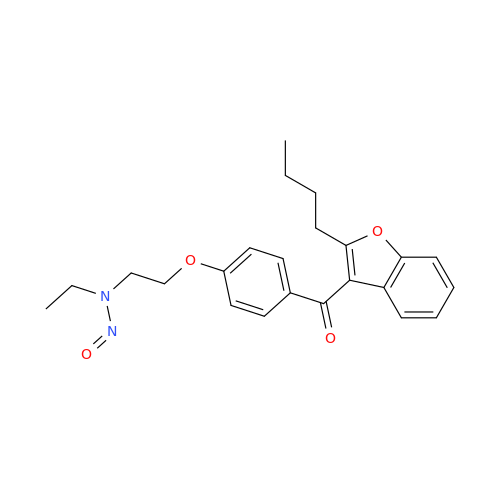
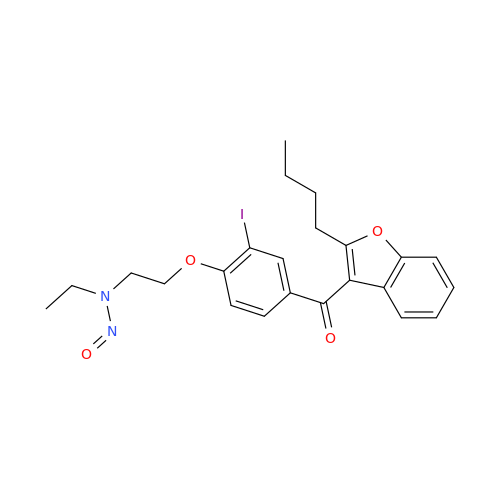
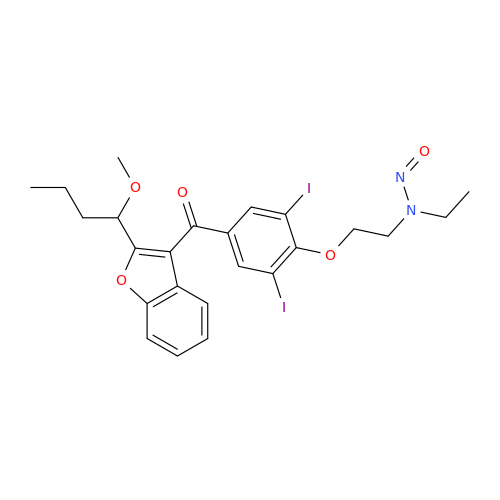
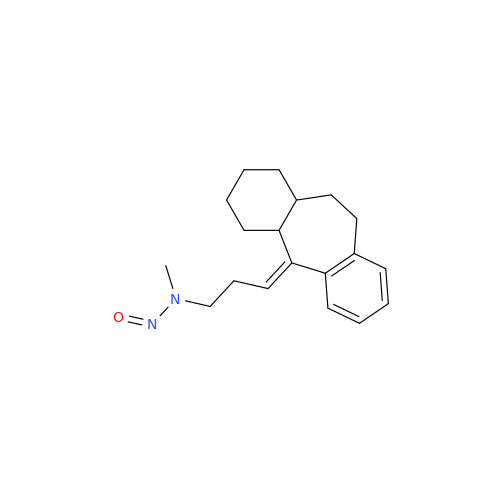
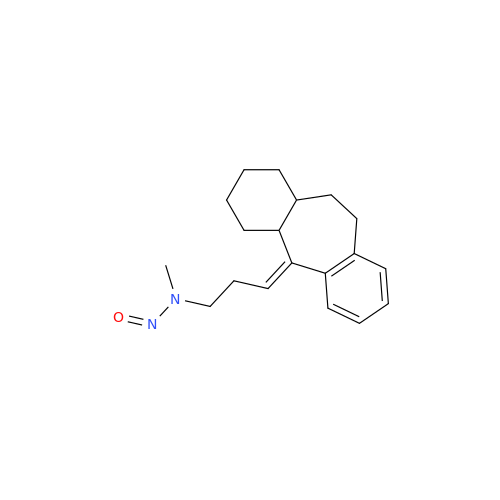
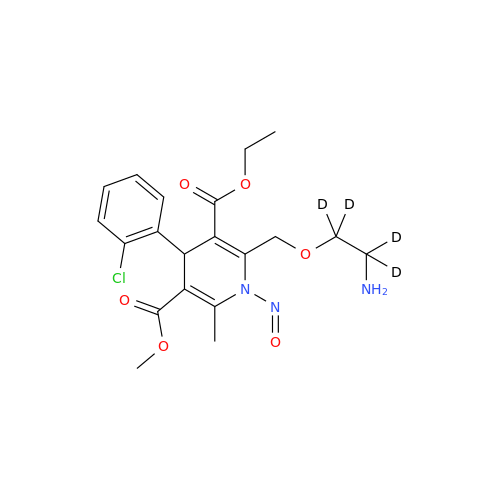
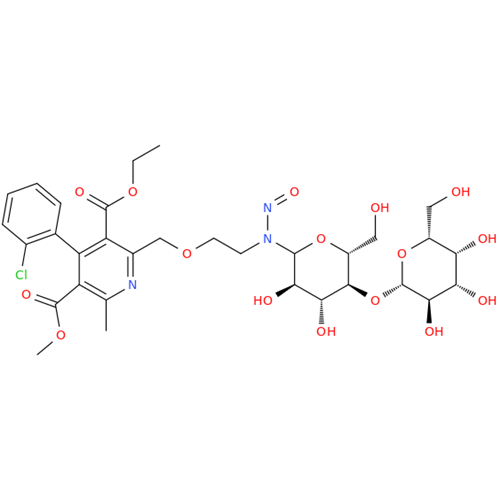
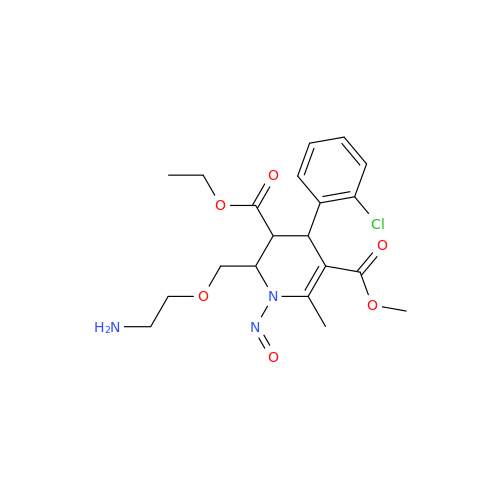
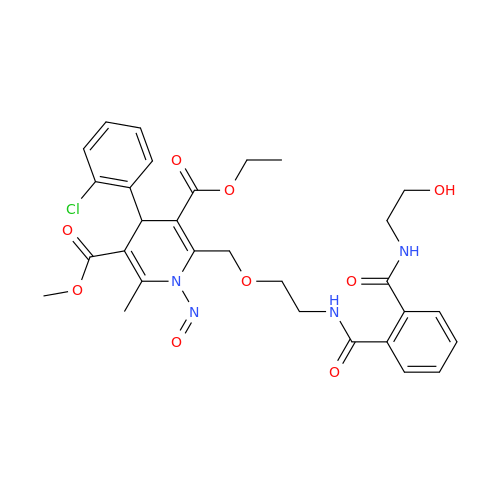
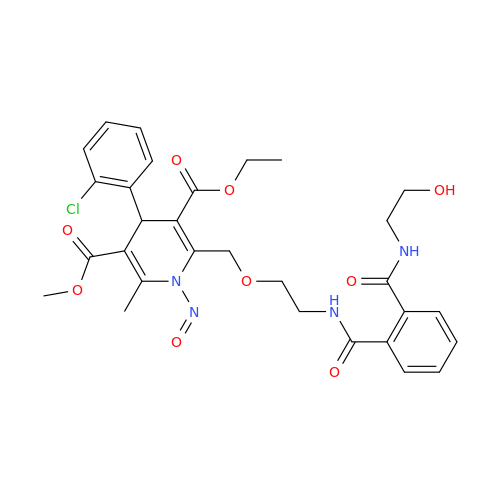
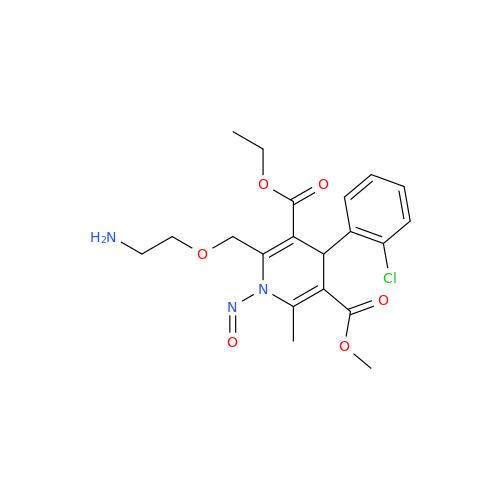
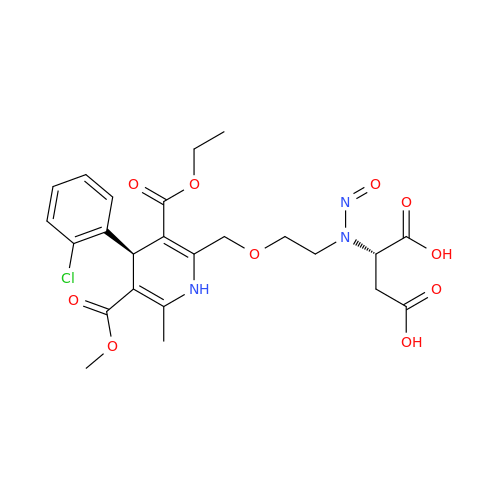
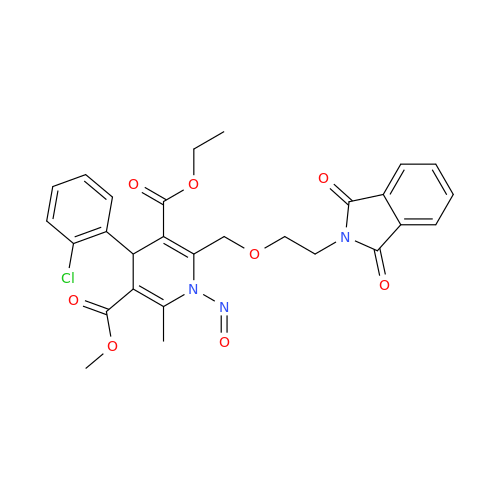
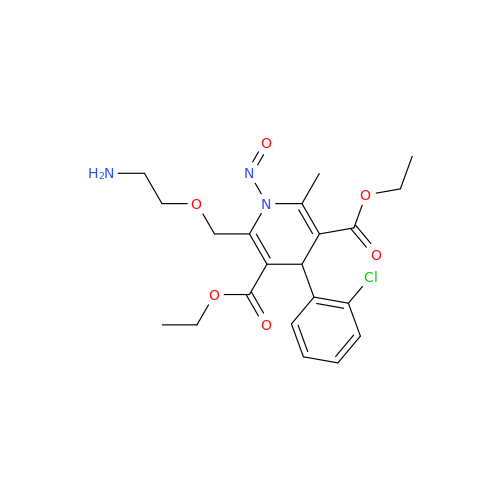
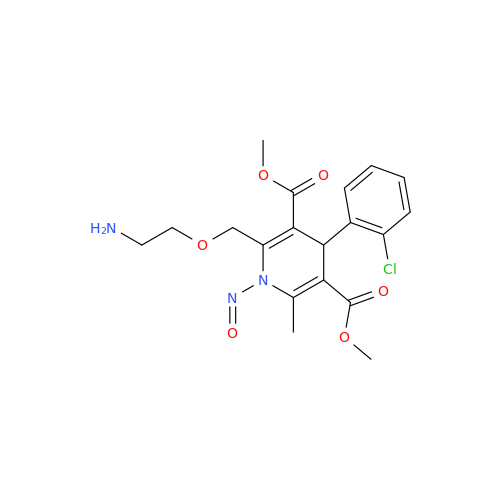
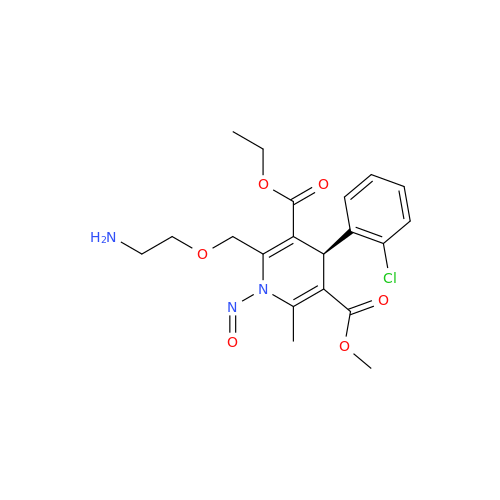
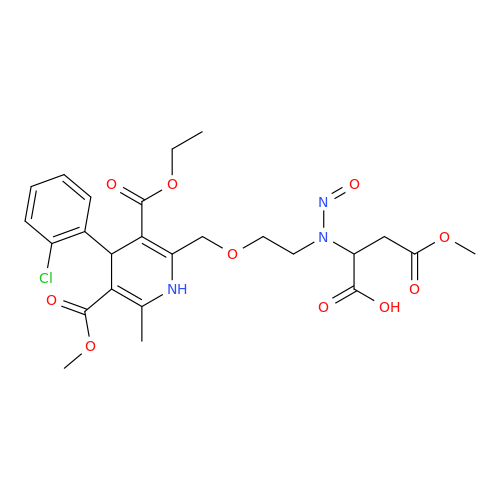
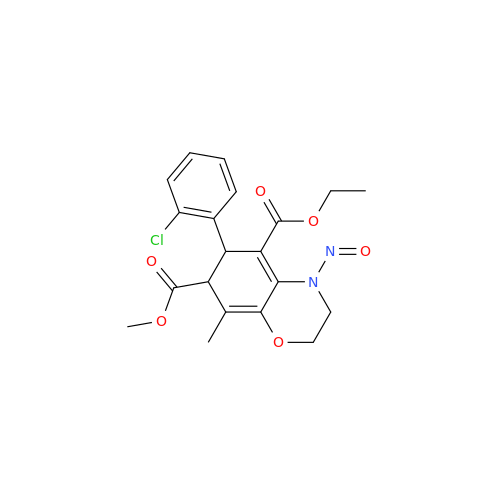
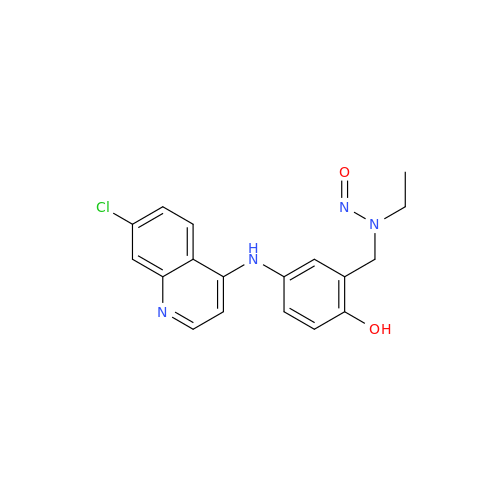
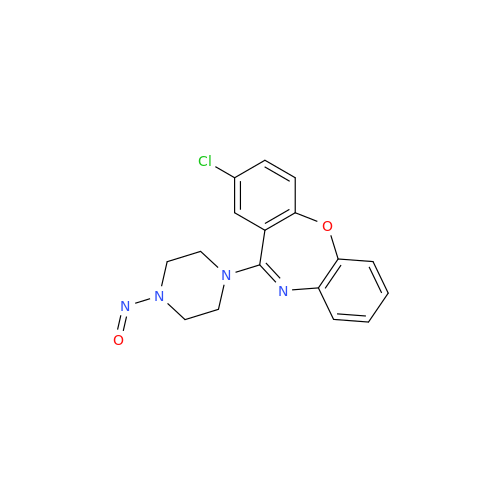
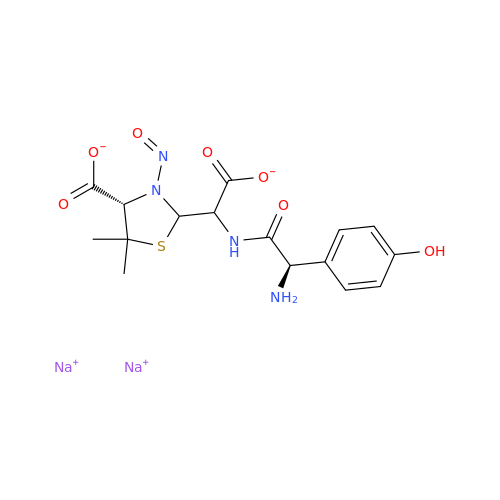
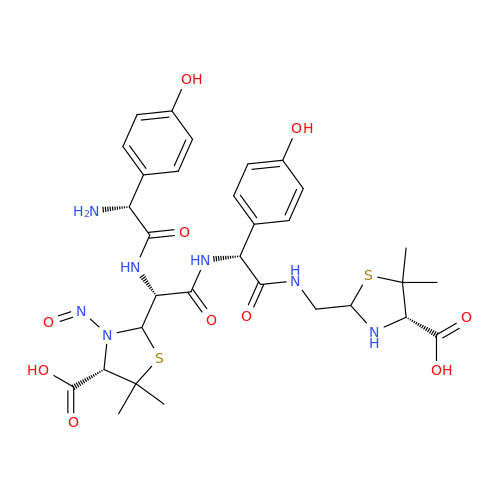
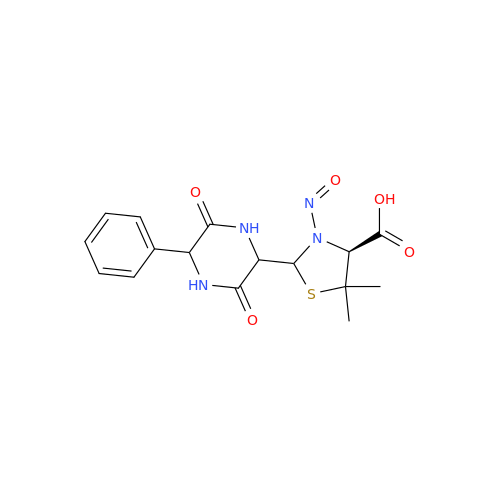
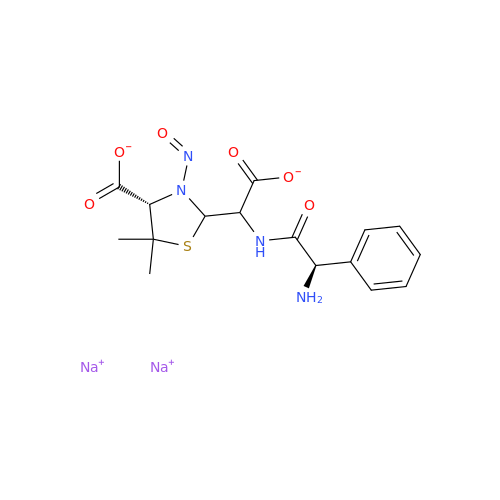
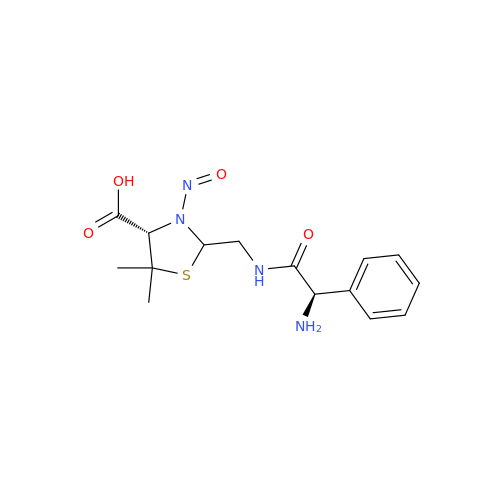
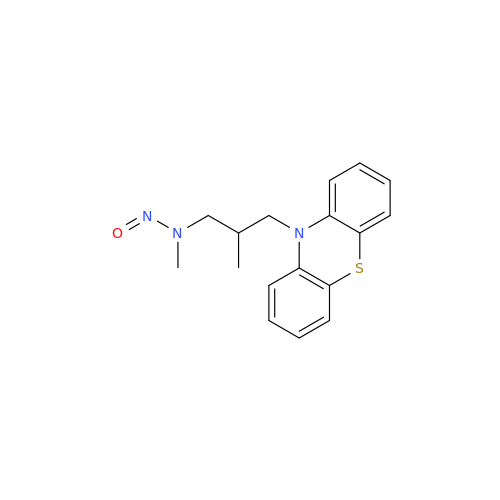
N-Nitoso Alimemazine EP Impurity B
Add to Cart
Bulk Enquiry
|
Chemical Name: N-Nitoso Alimemazine EP Impurity B
Synonym: N-Methyl-N-(2-methyl-3-(10H-phenothiazin-10-yl)propyl)nitrous amide