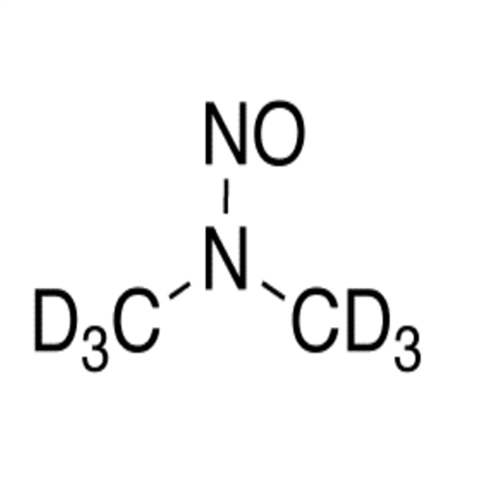
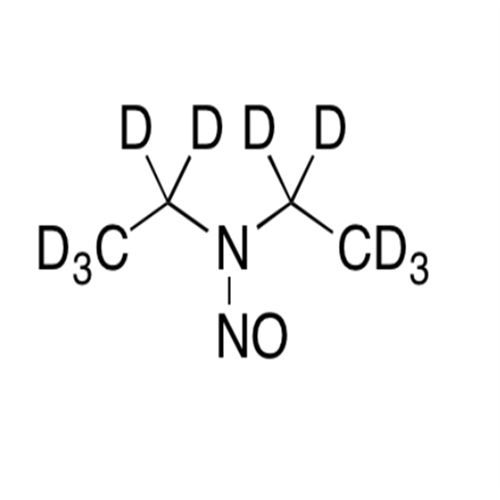
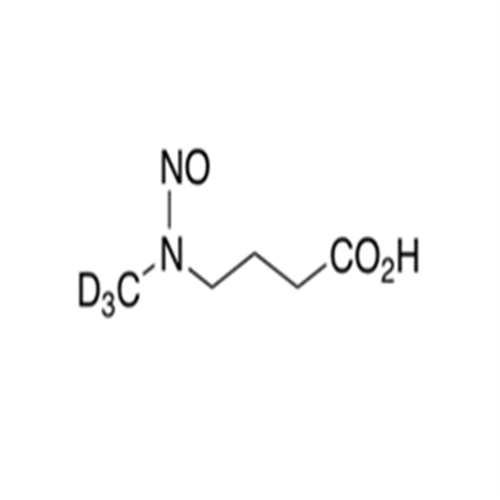
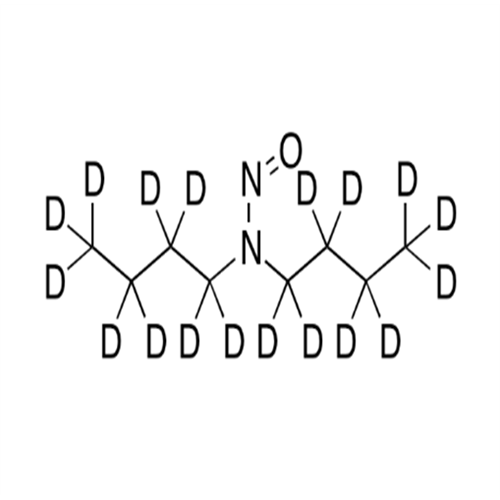
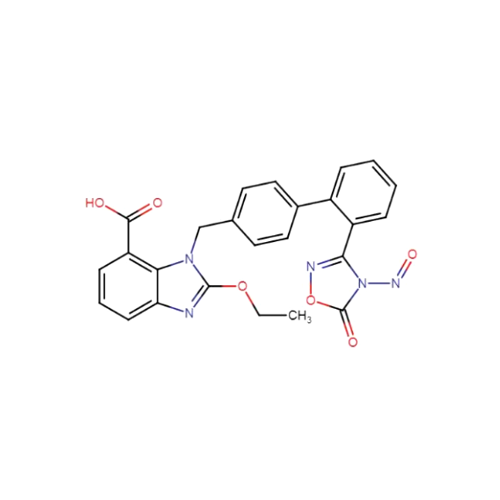
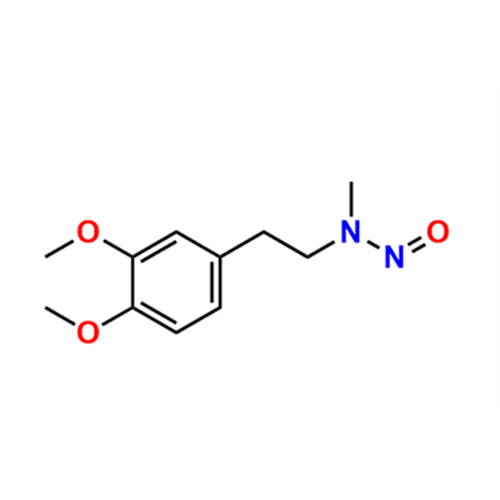
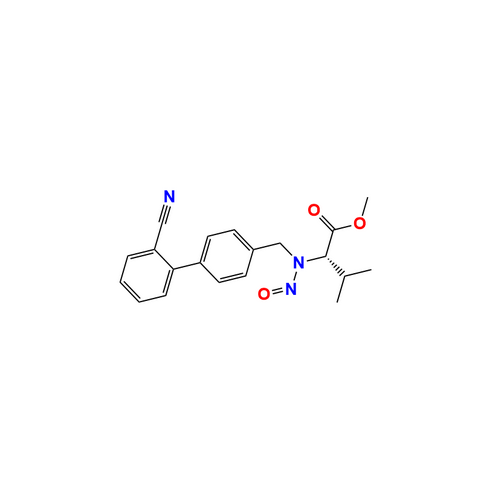
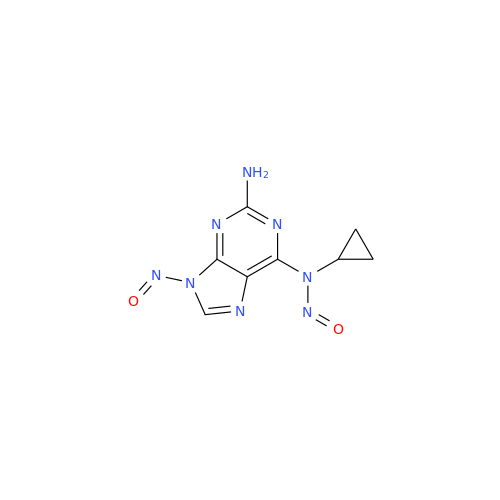
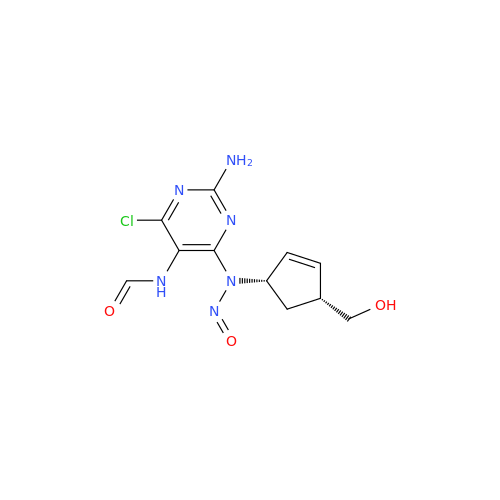
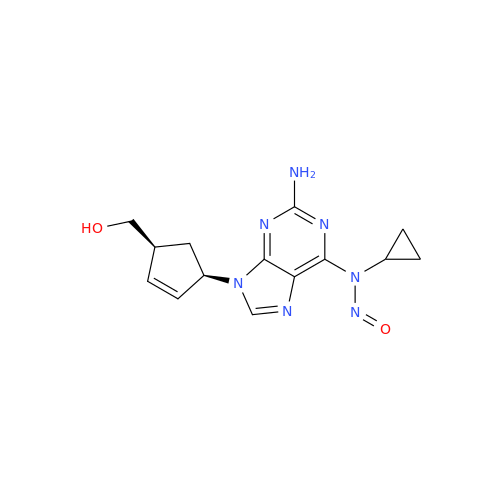
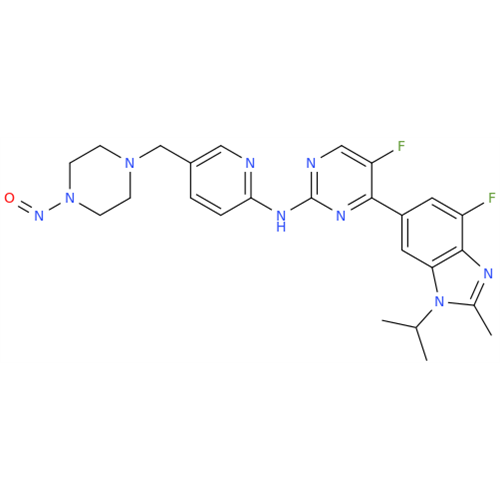
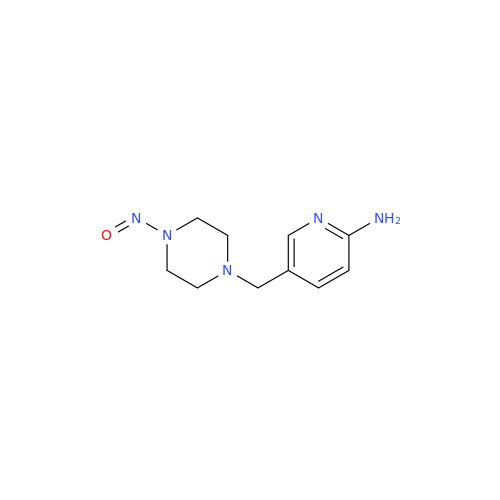
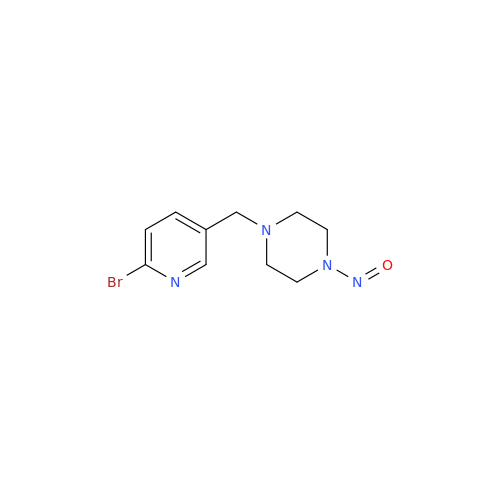
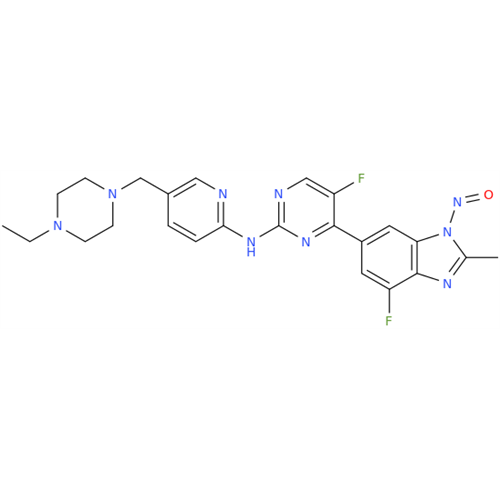
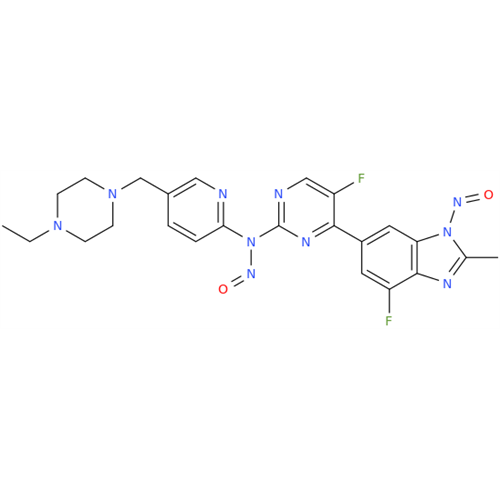
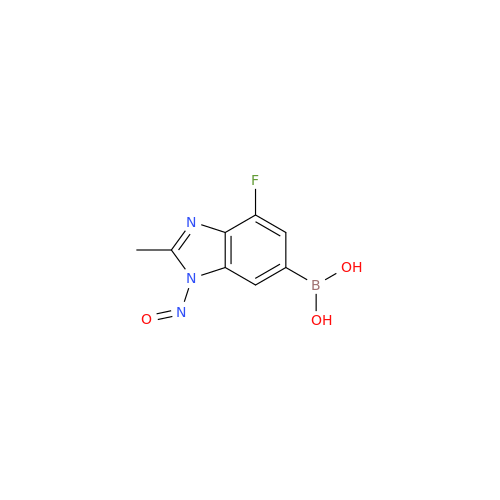
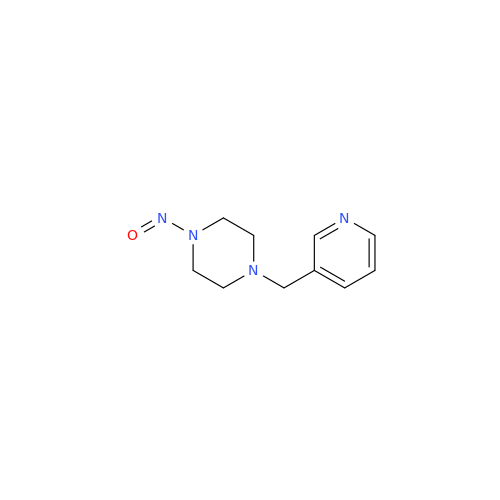
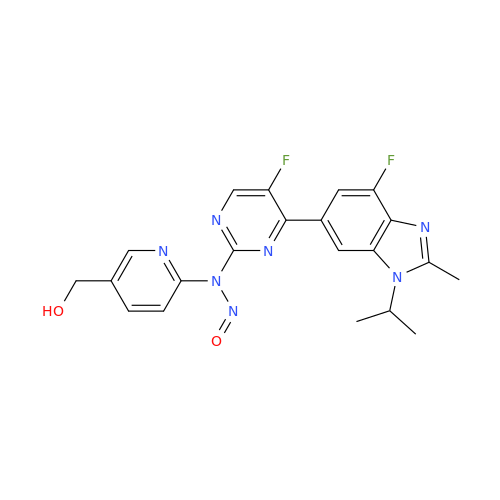
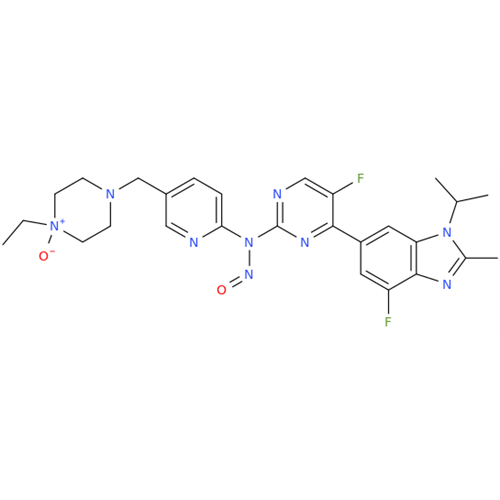
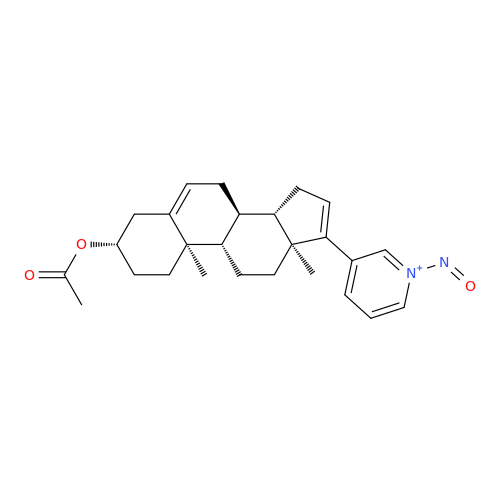
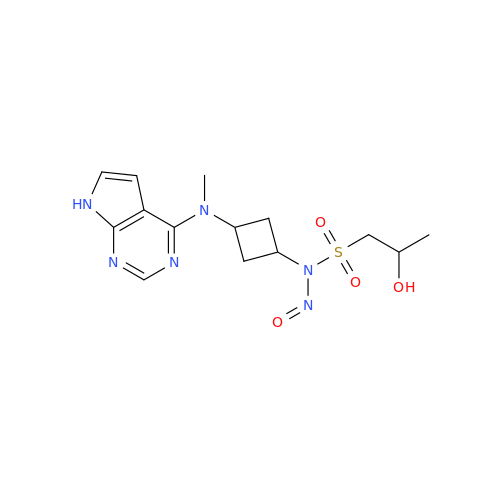
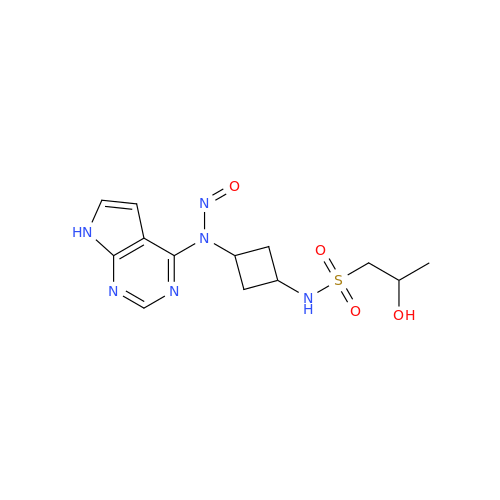
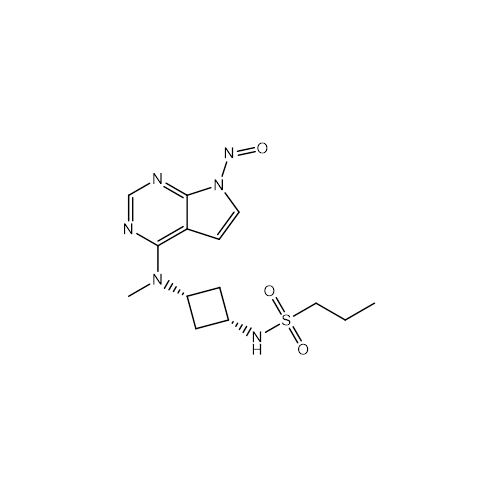
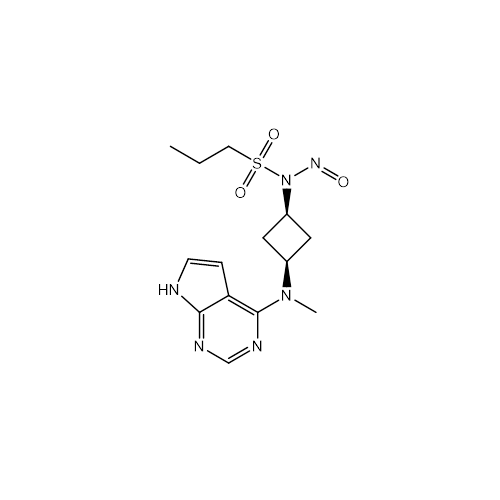
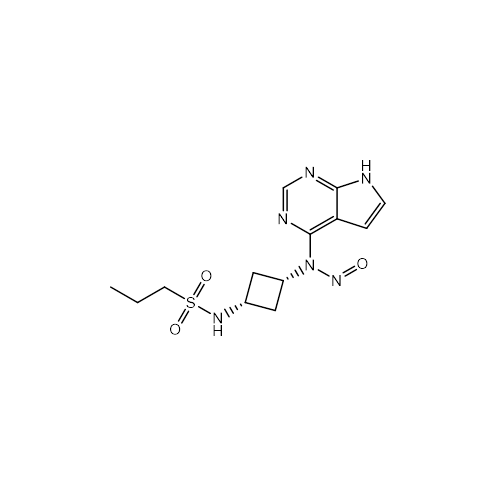
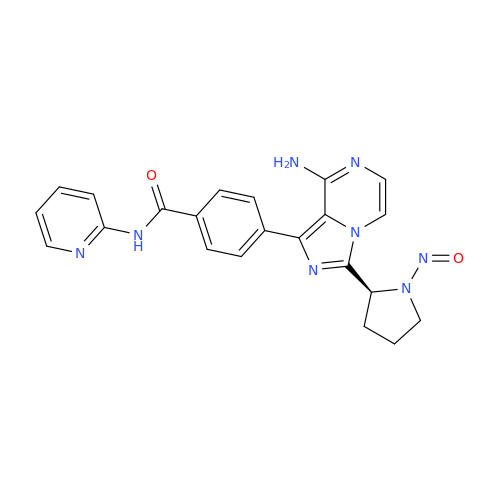
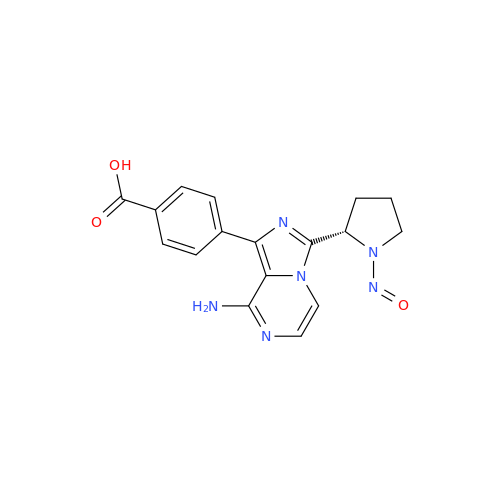
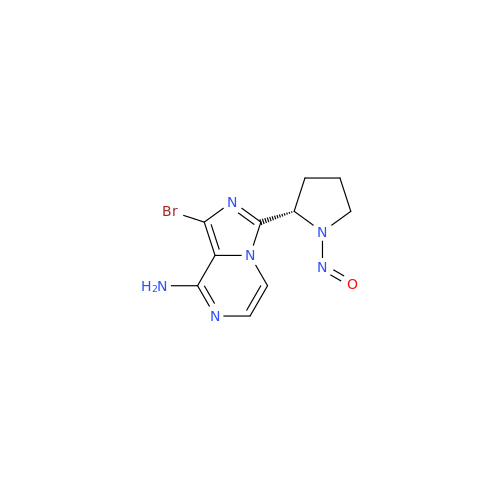
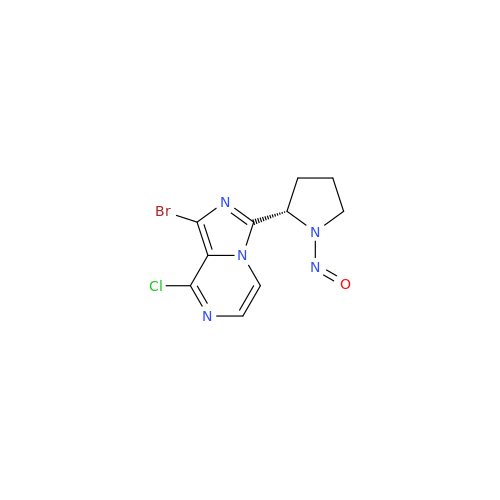
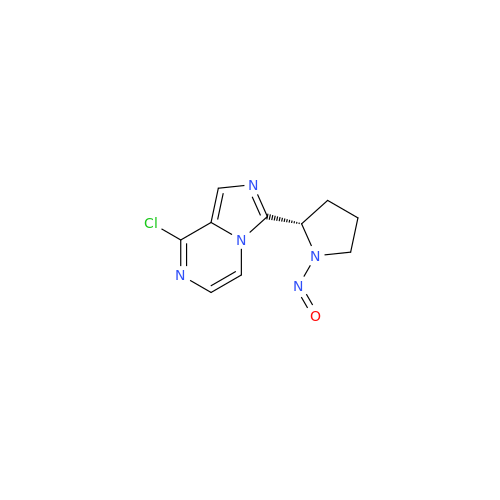
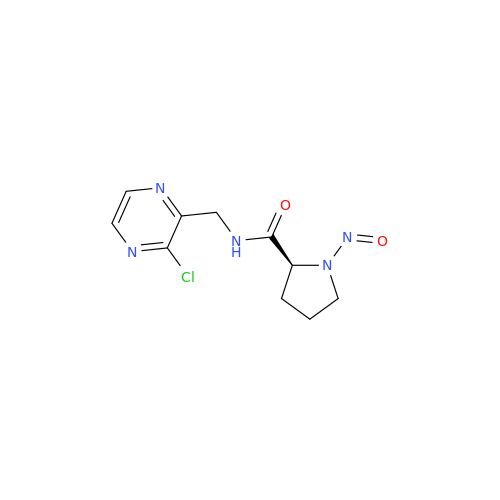
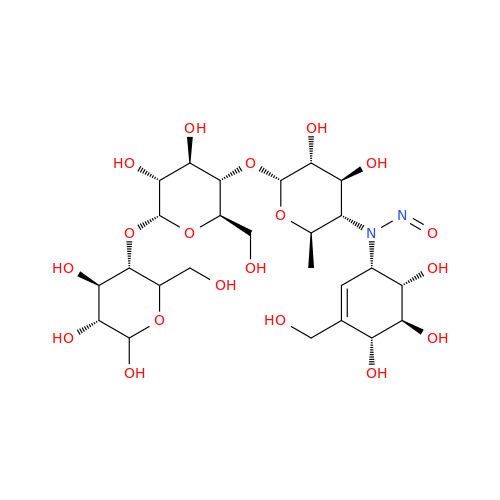
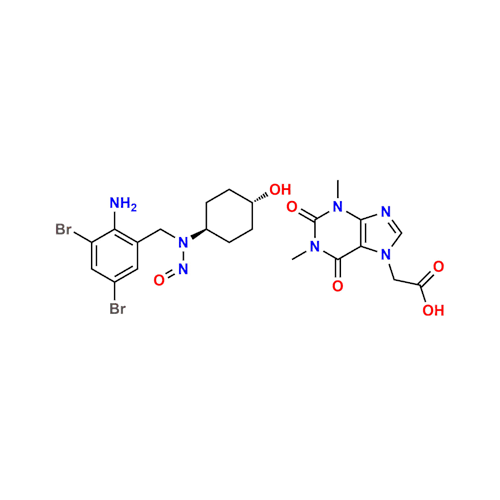
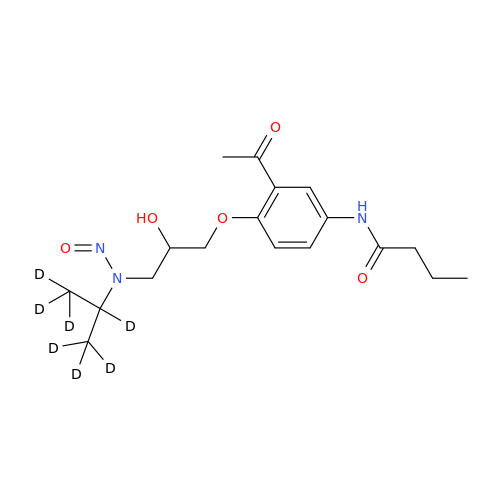
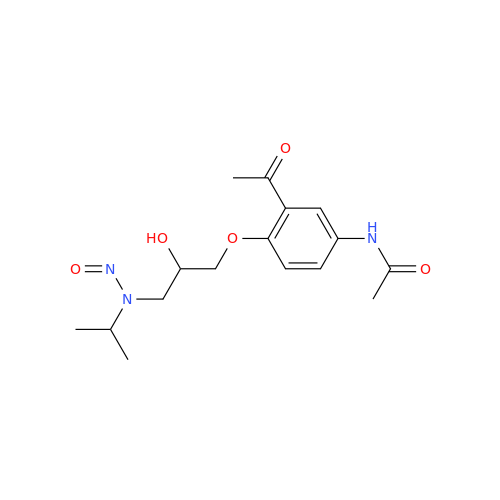
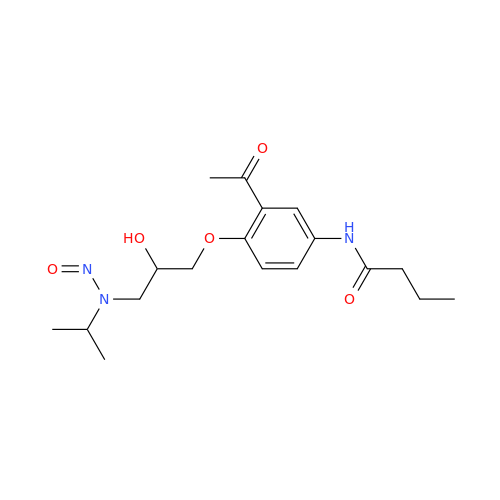
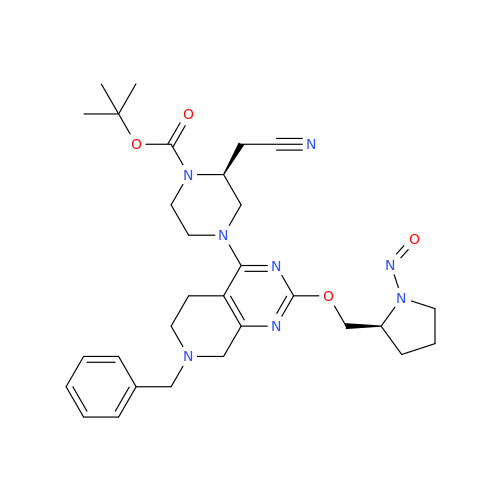
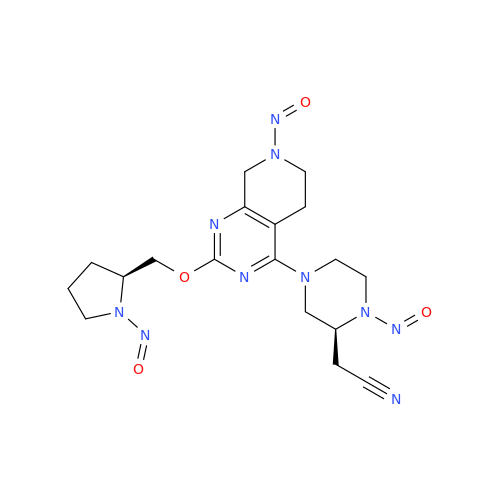
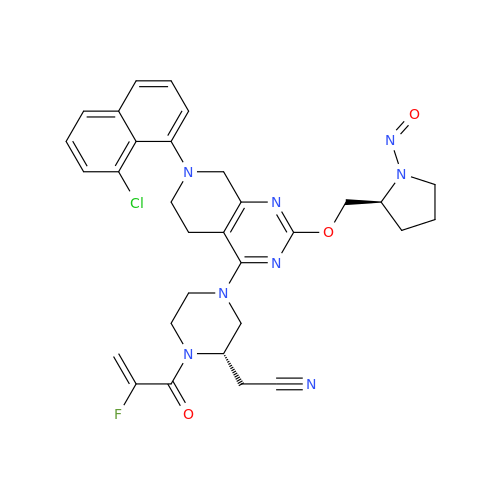
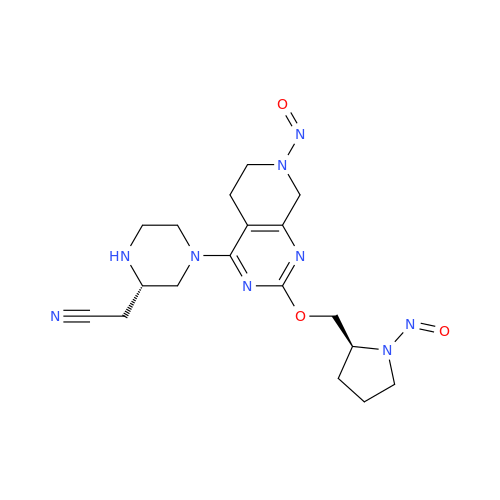
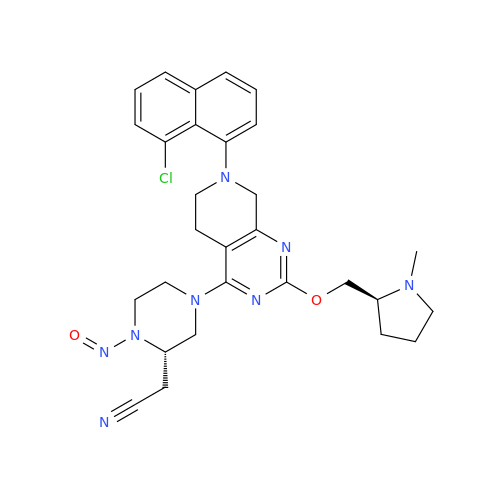
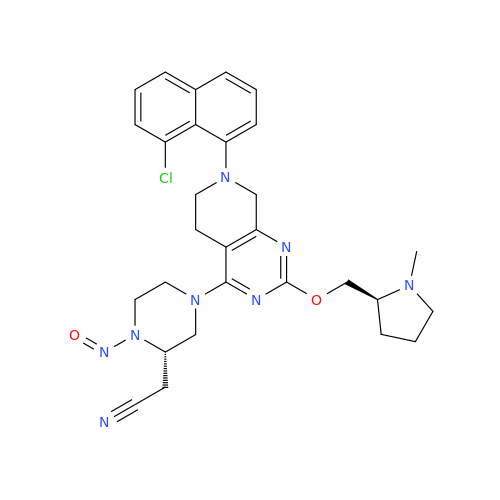
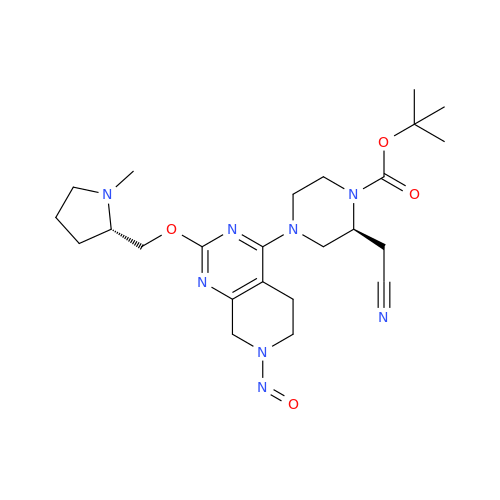
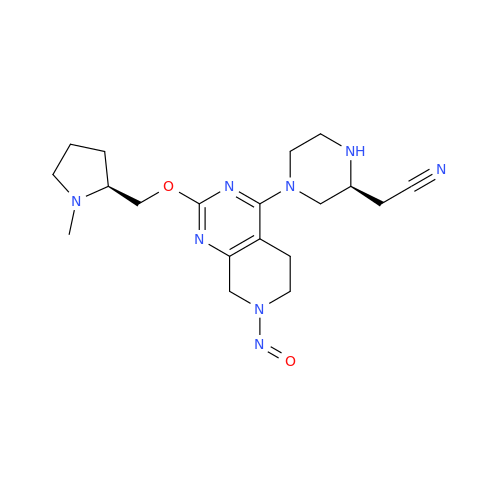
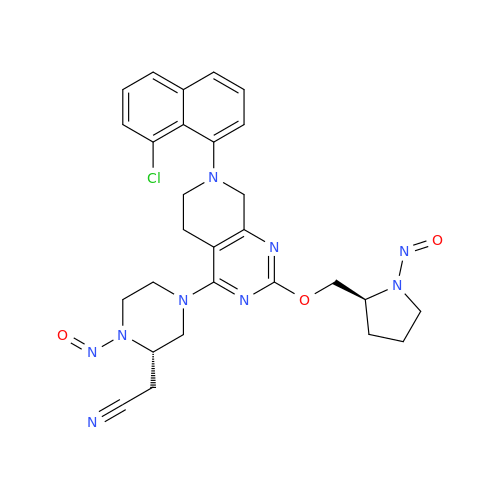
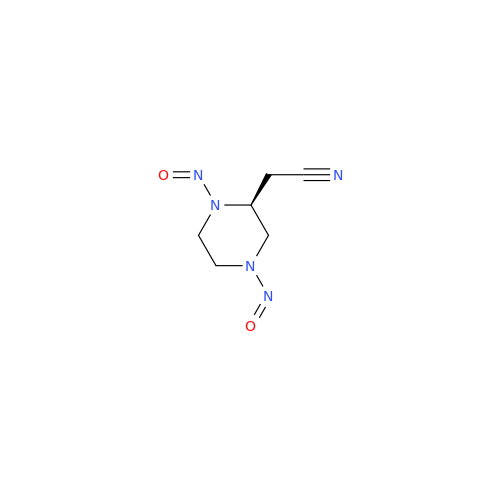
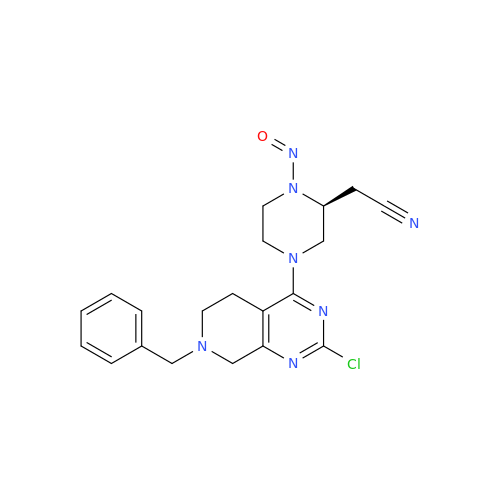
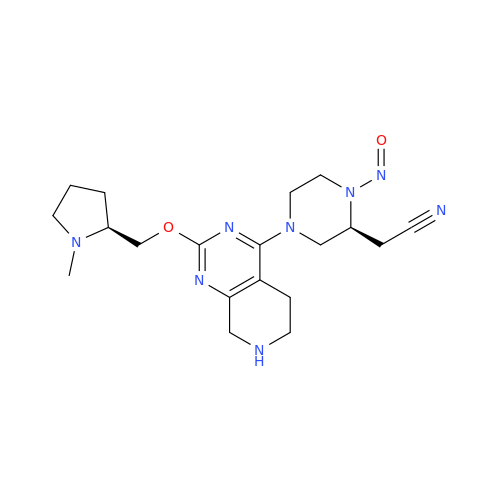
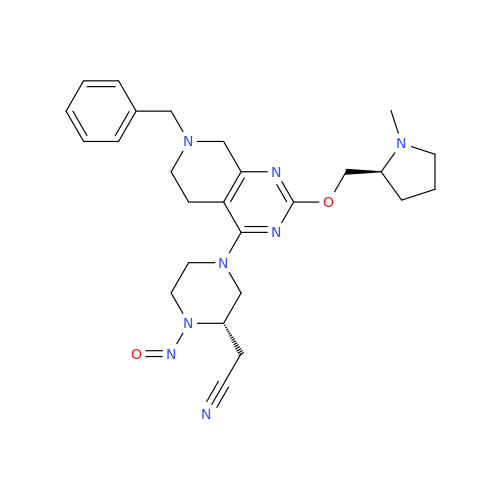
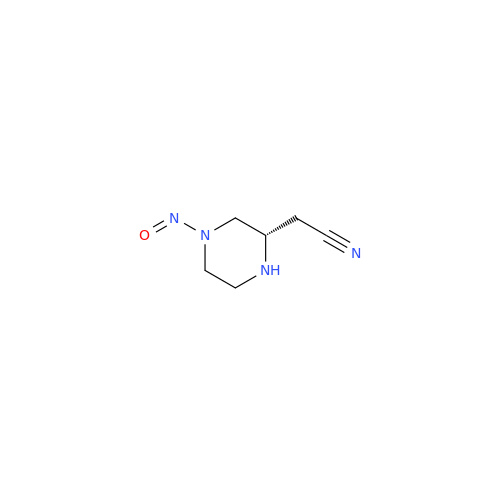
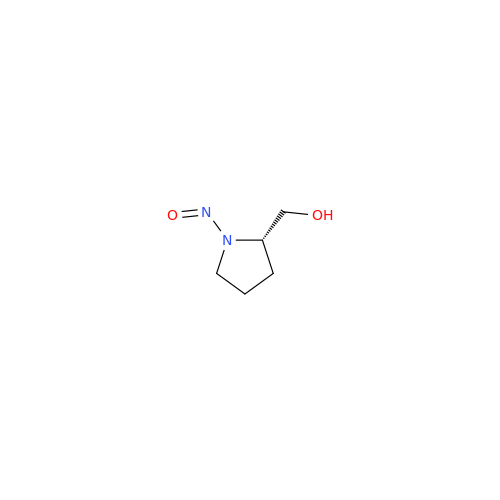
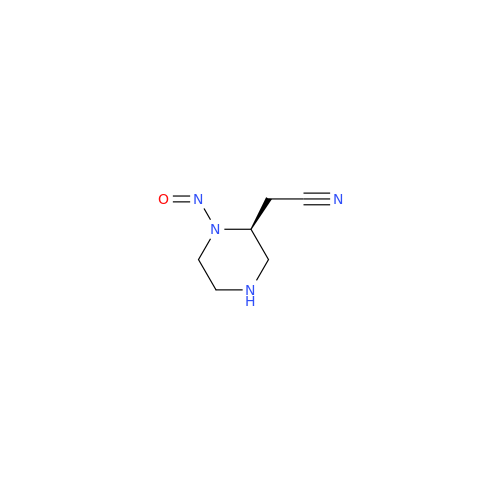
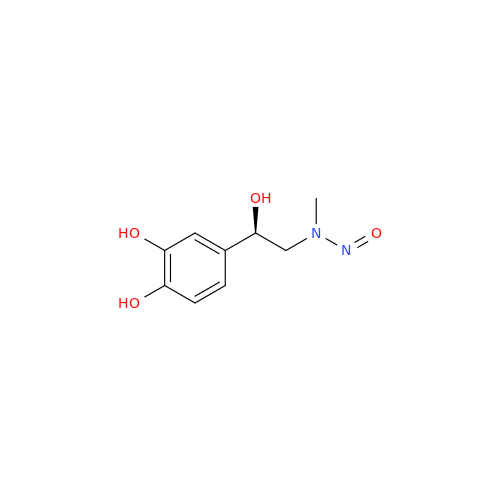
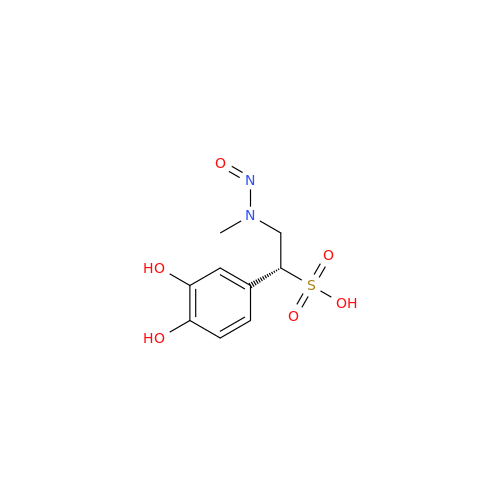
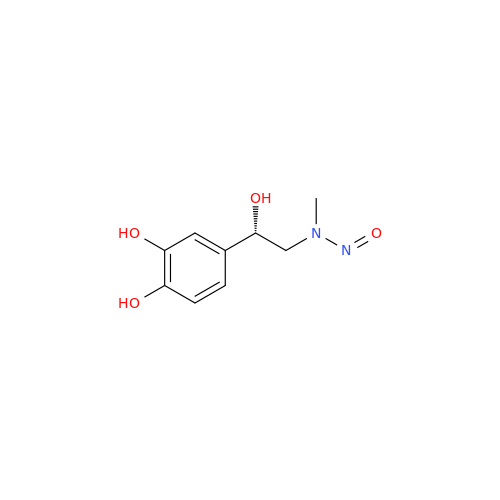
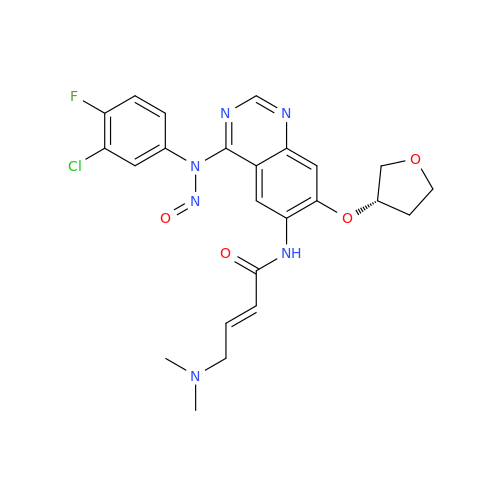
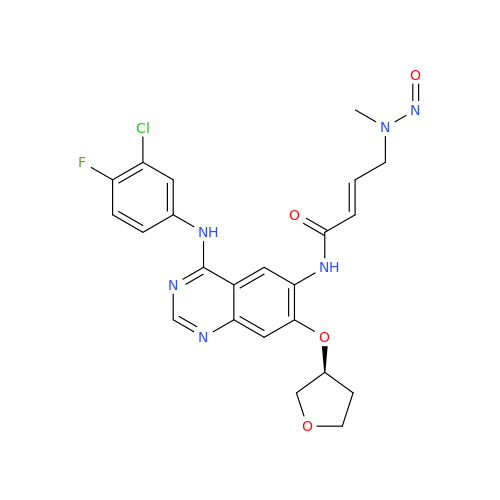
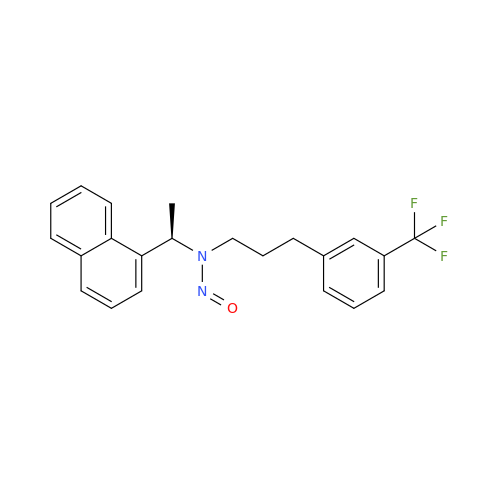
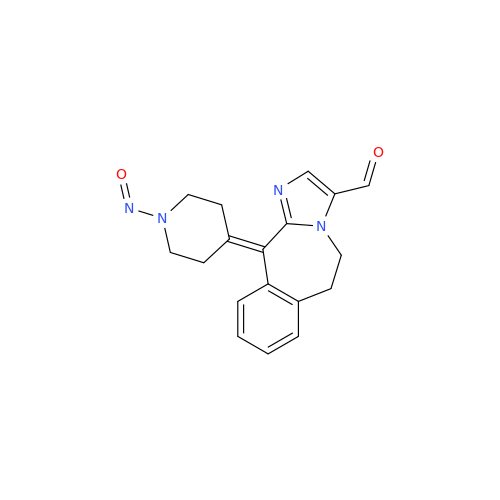
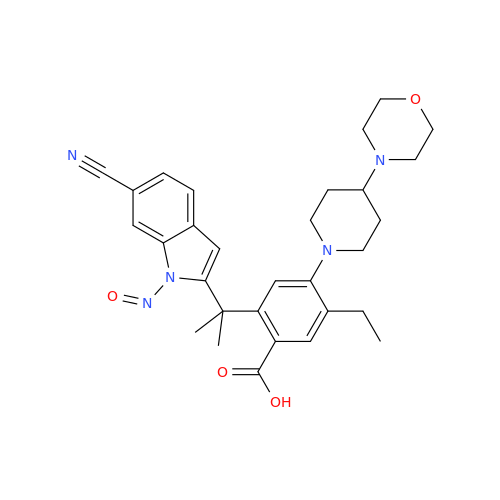
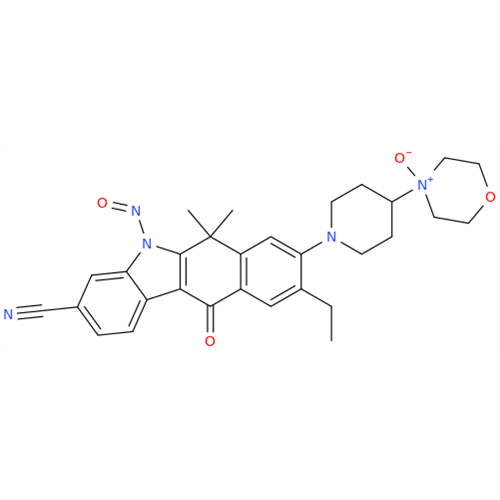
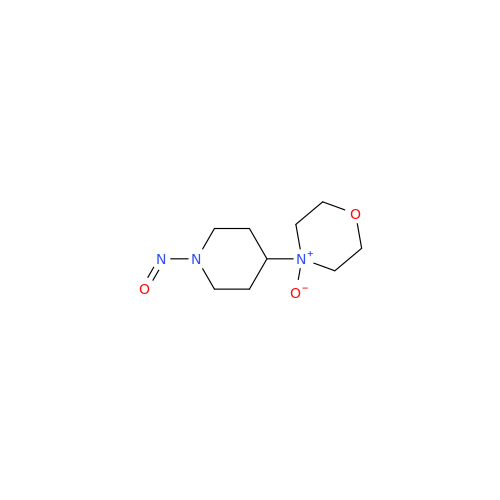
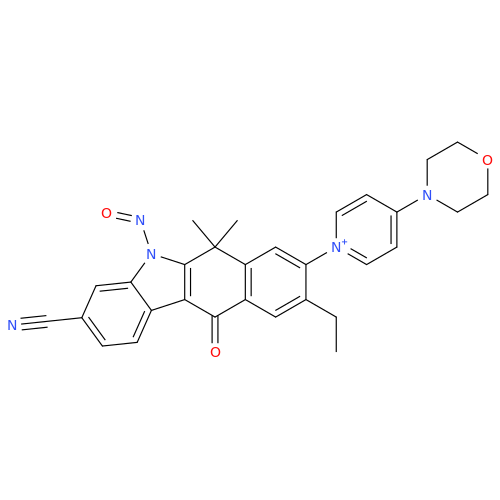
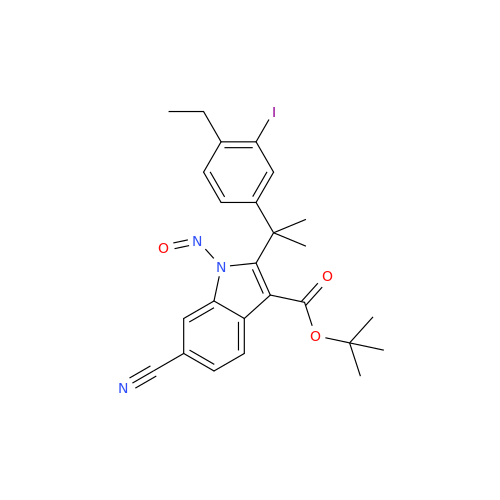
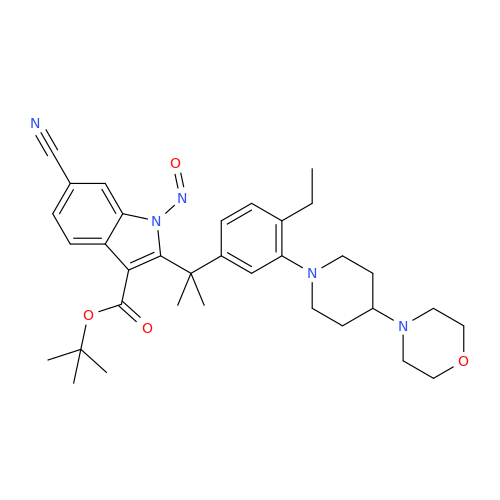
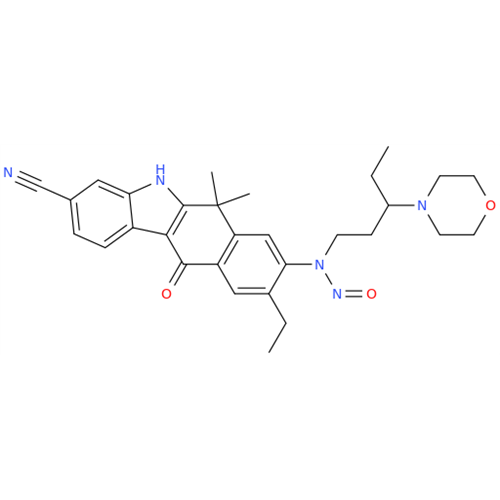
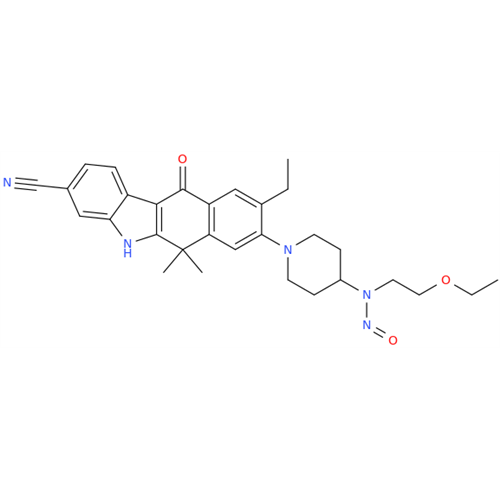
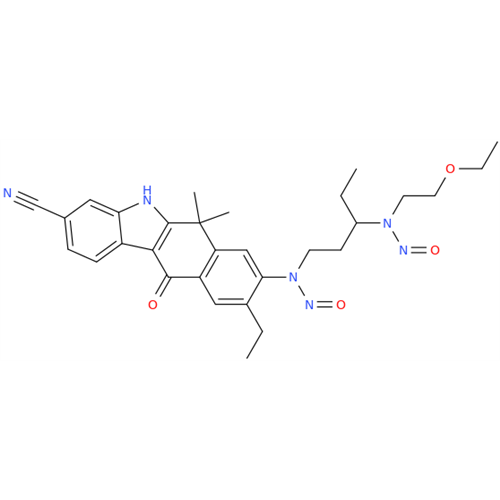
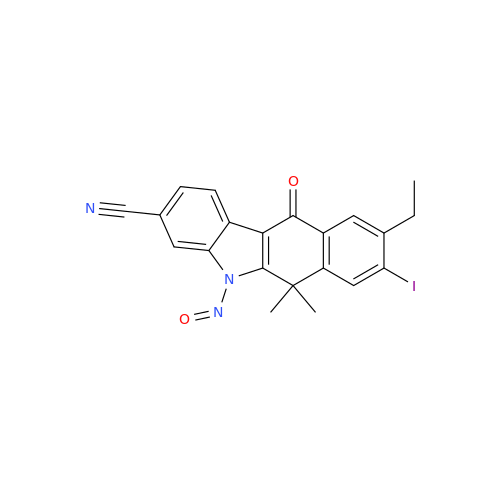
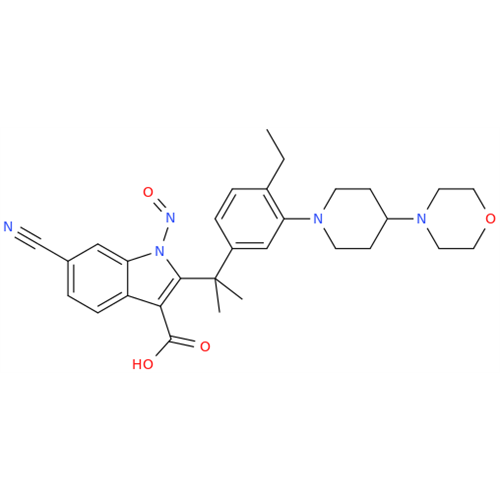
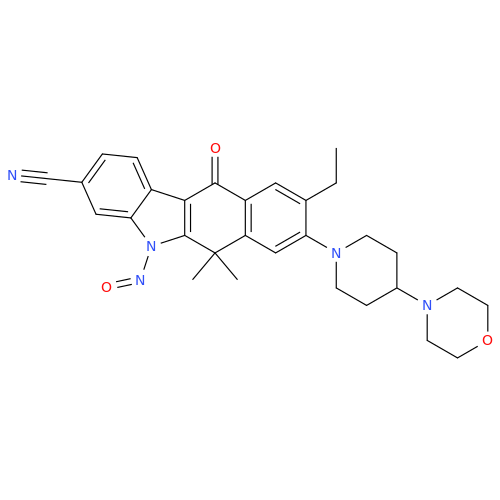
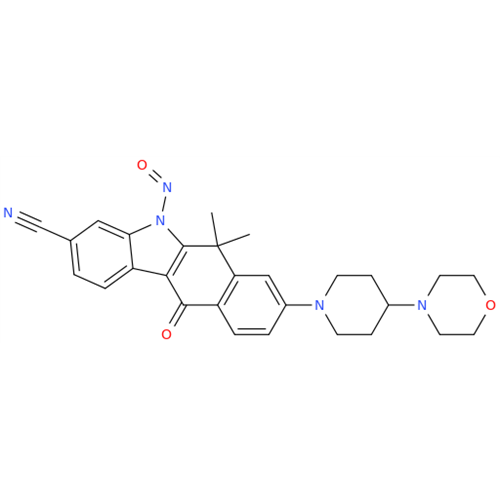
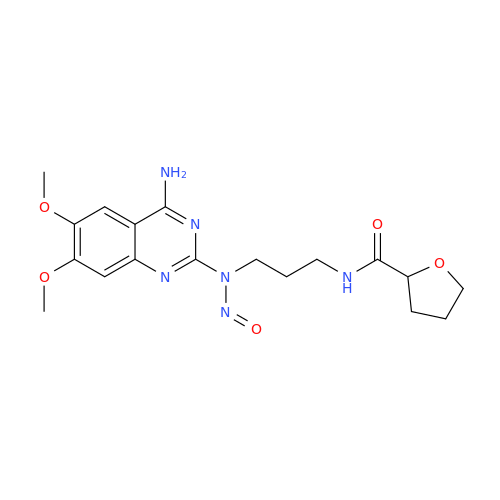
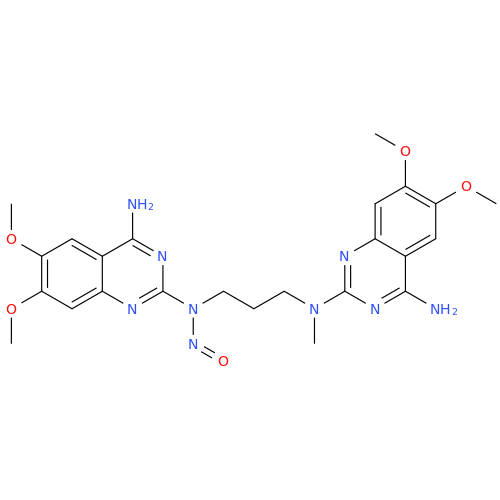
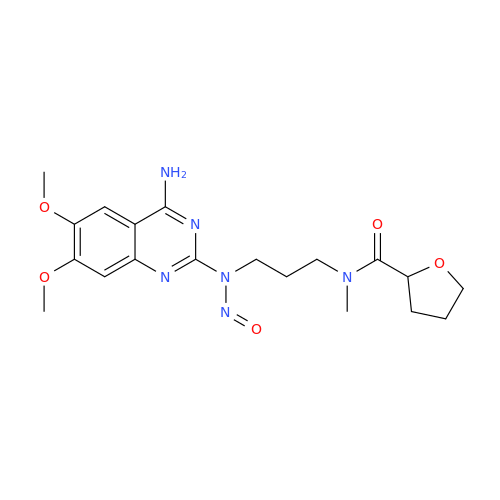
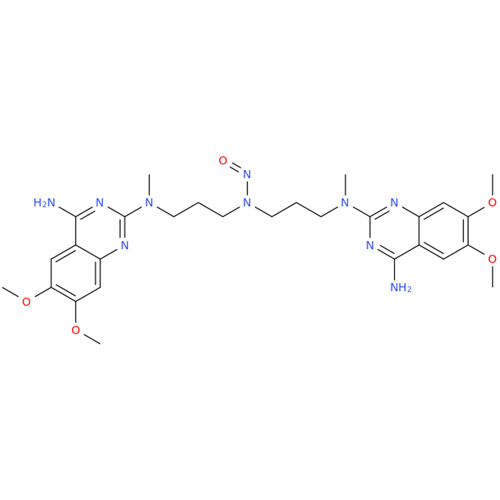
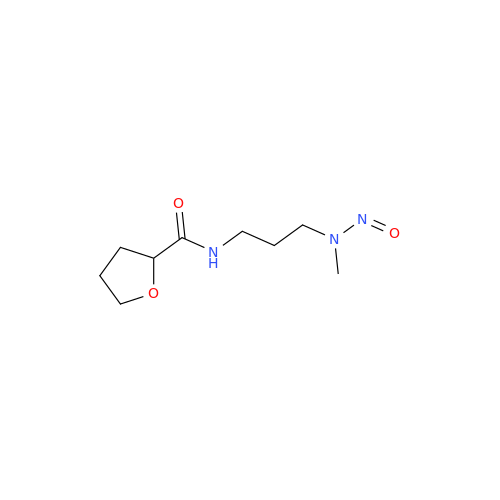
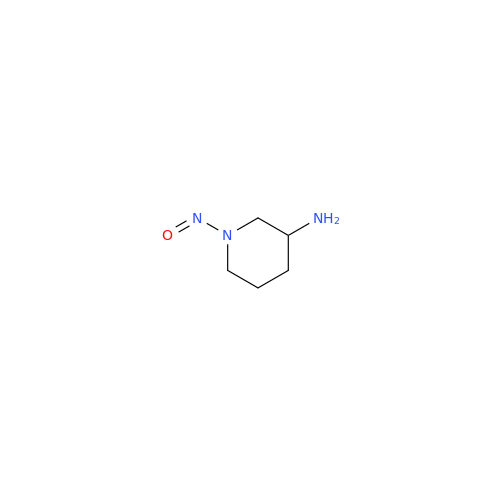
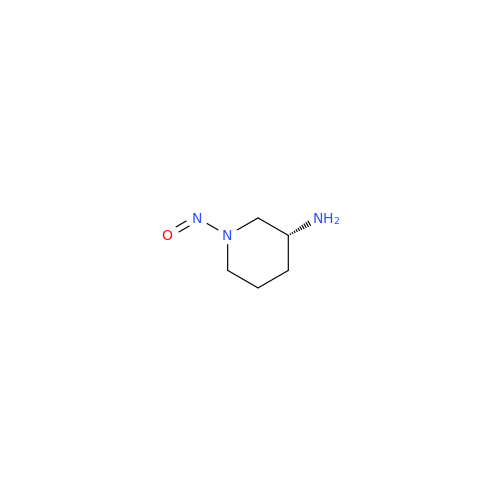
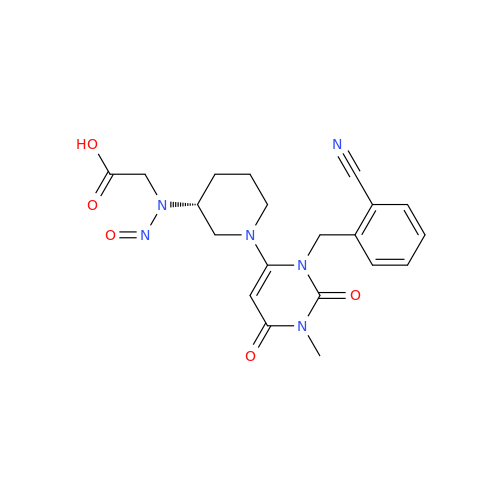
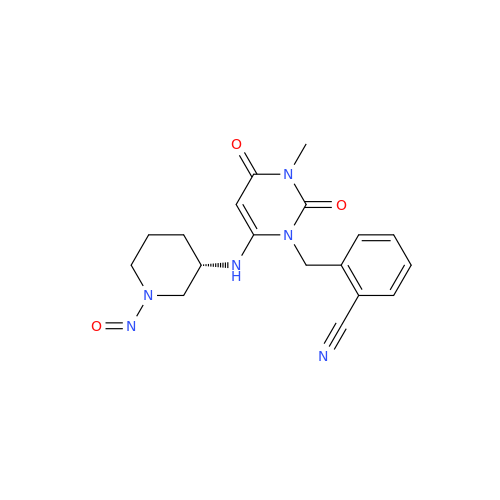
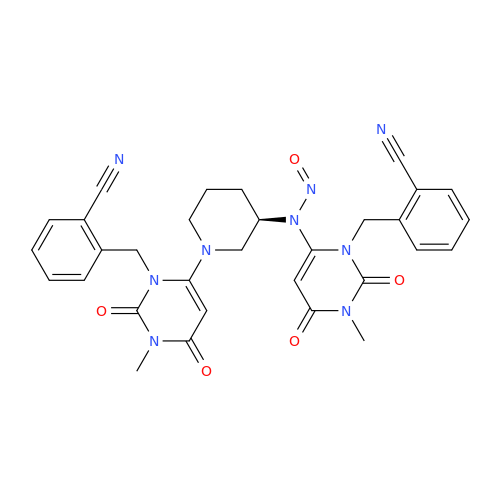
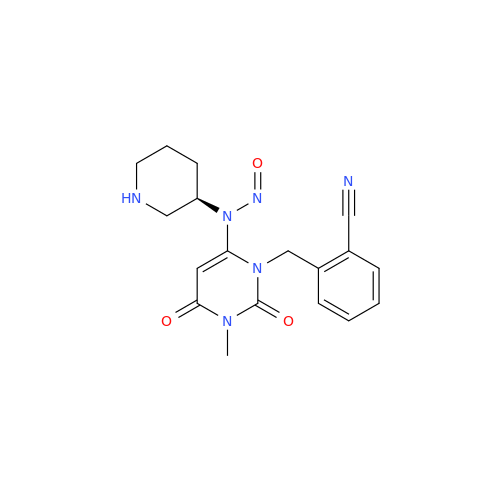
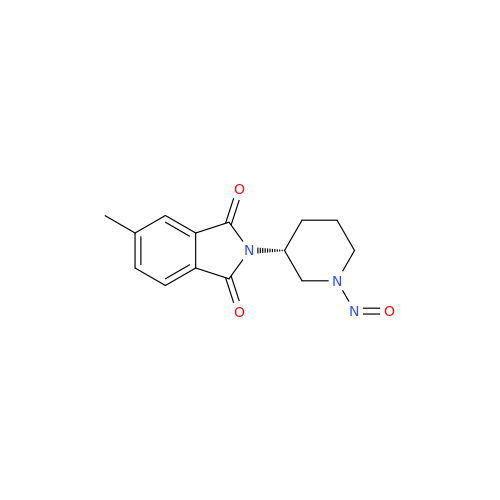
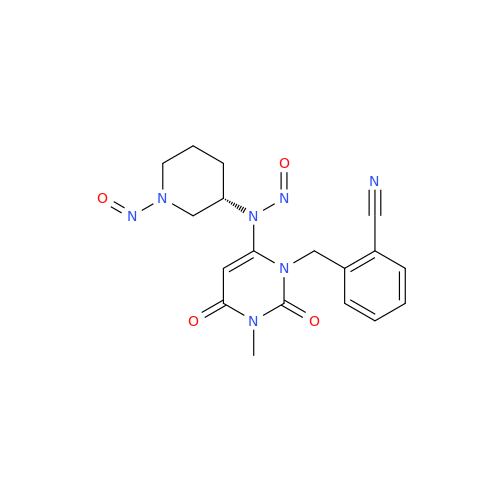
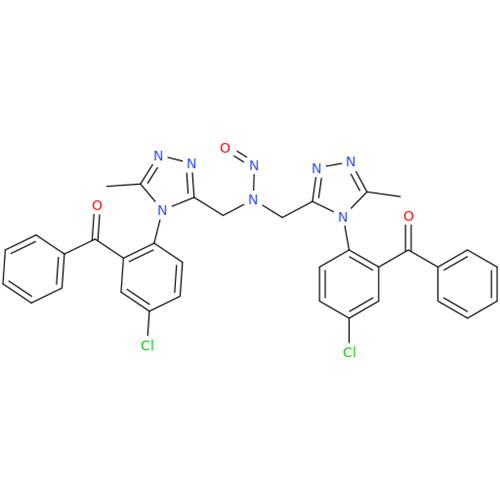
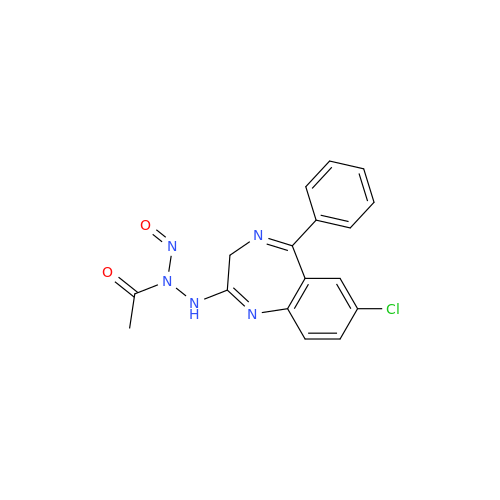
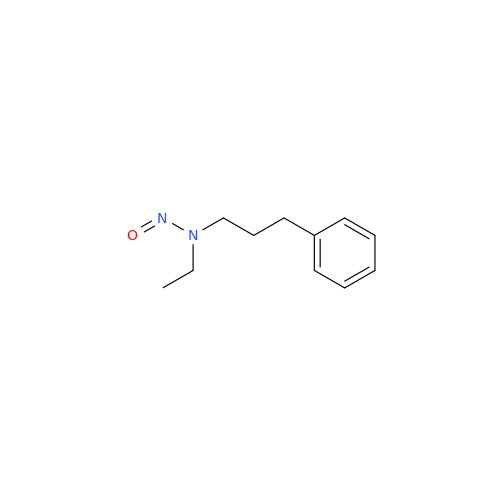
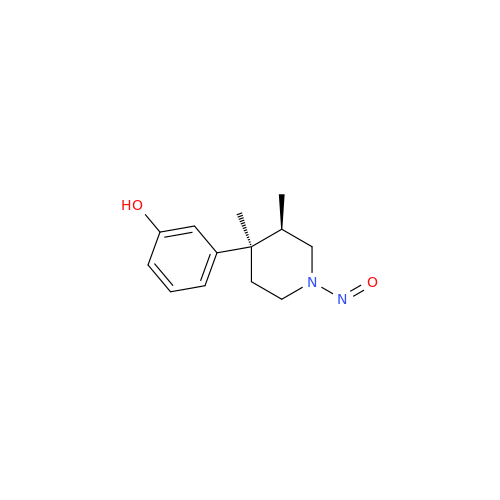
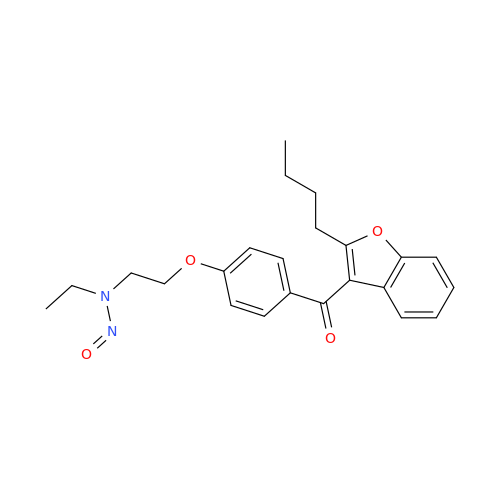
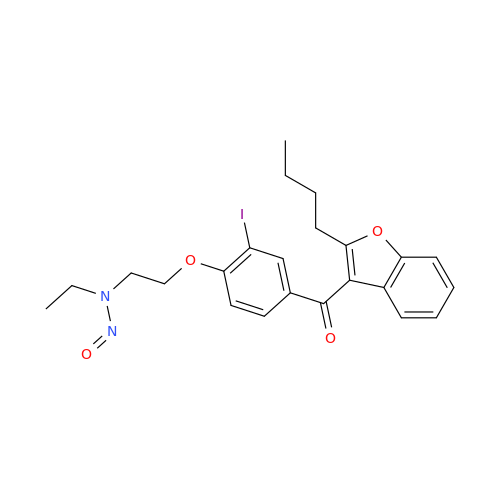
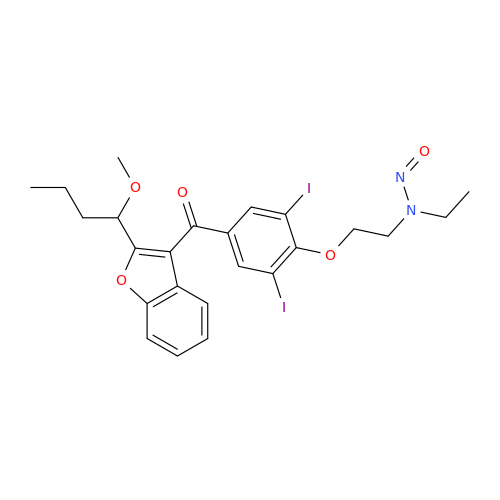
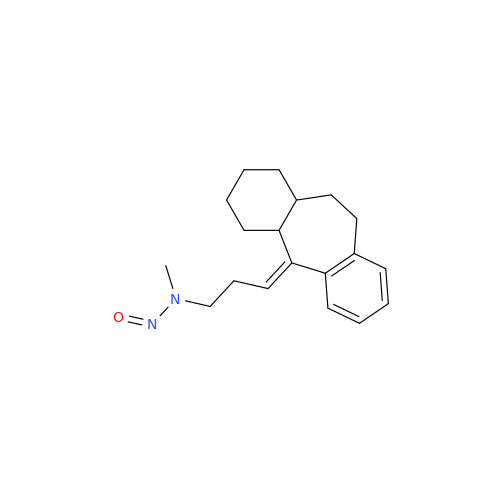
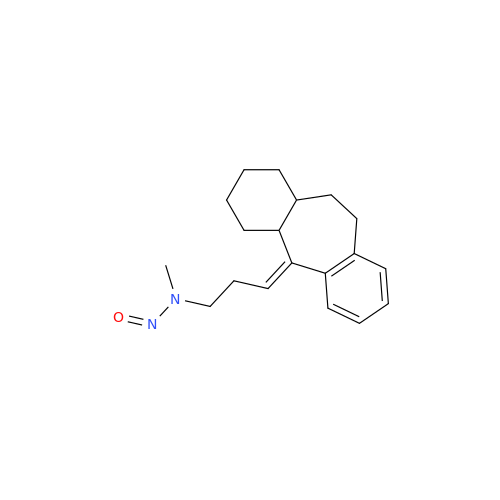
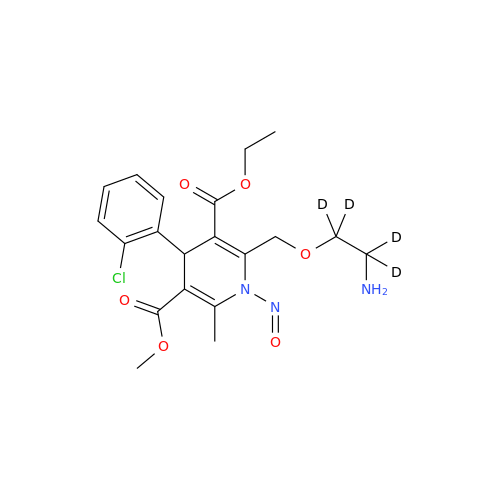
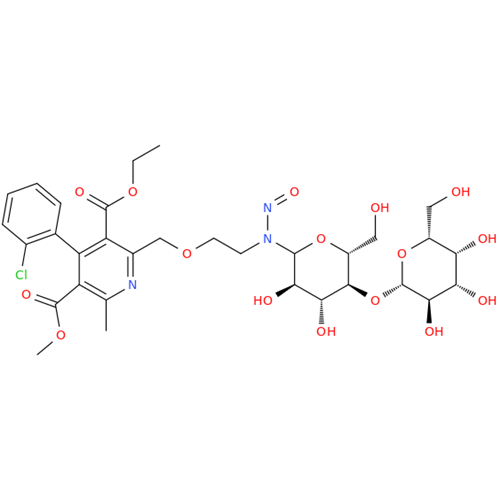
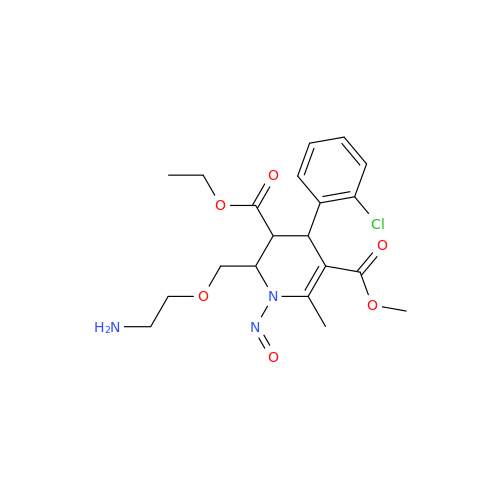
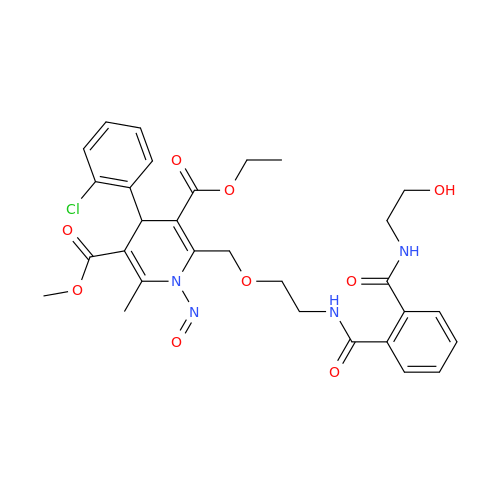
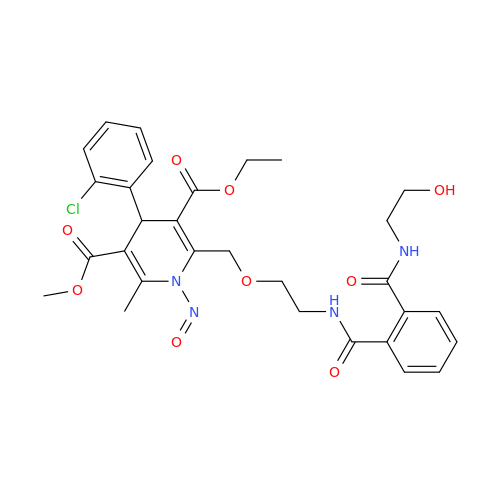
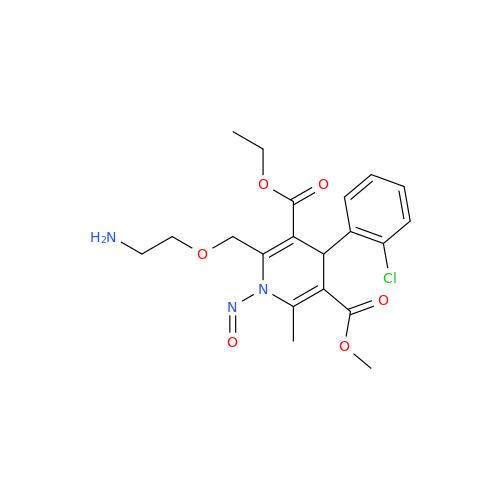
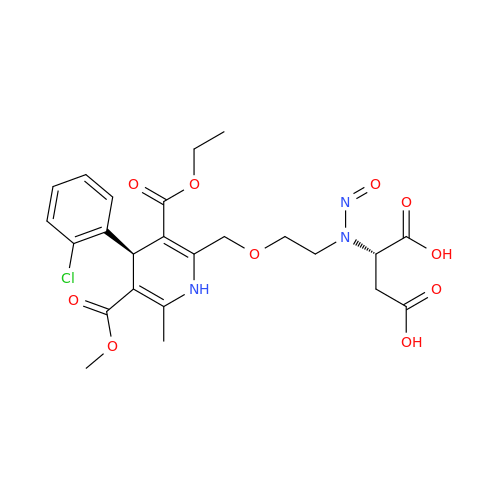
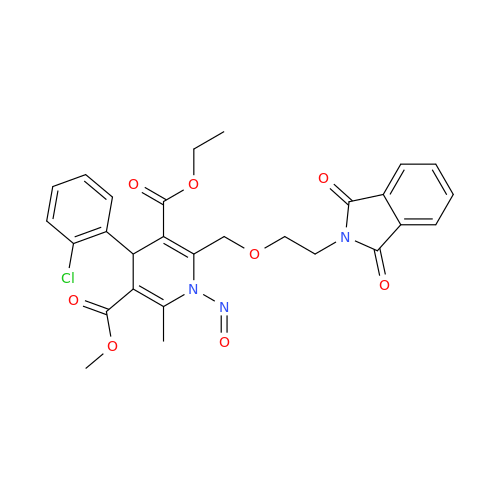
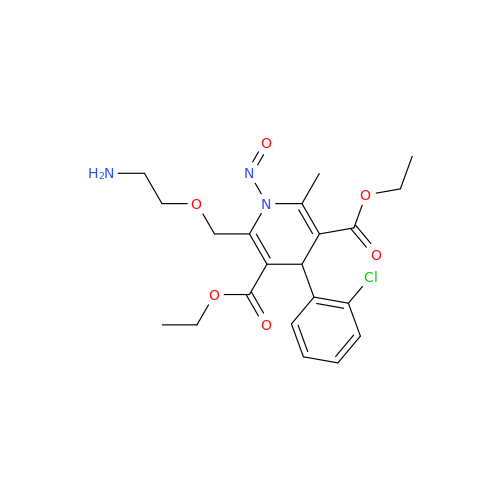
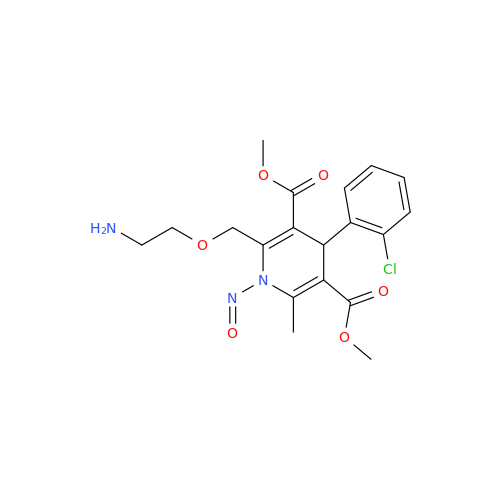
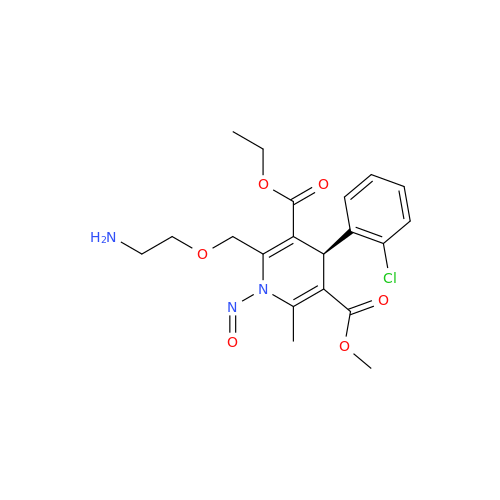
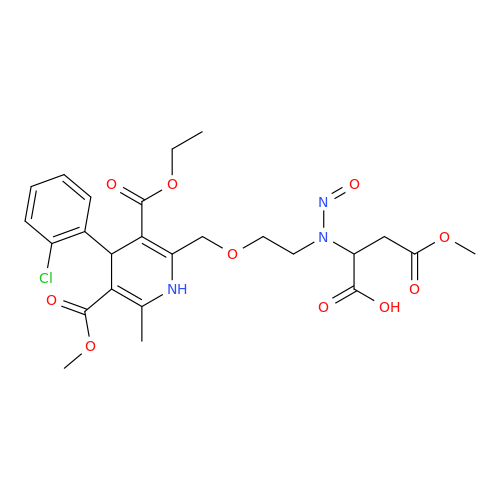
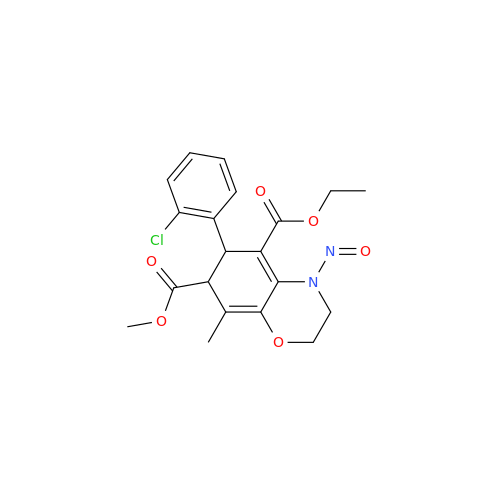
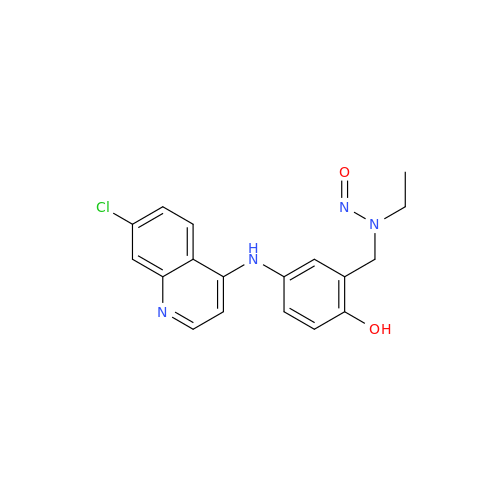
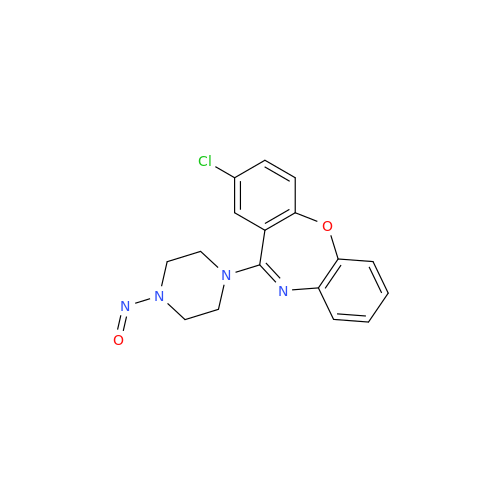
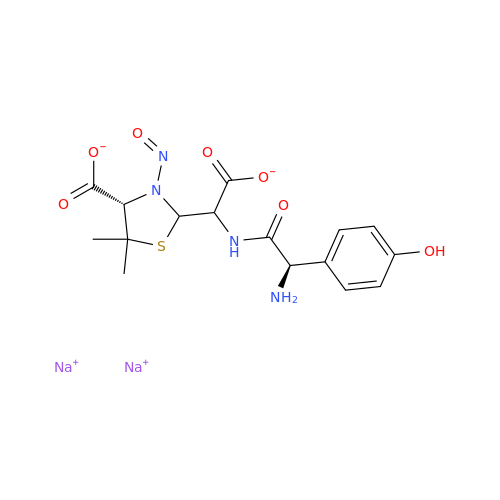
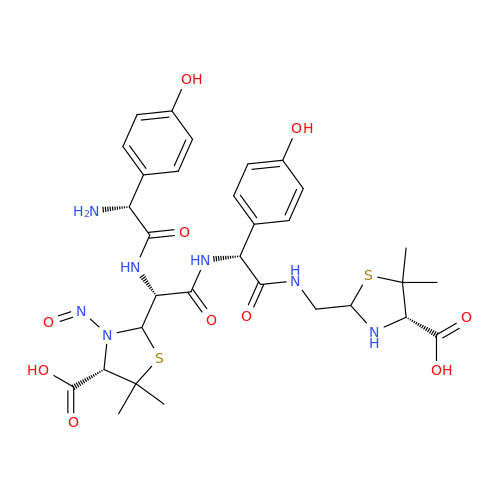
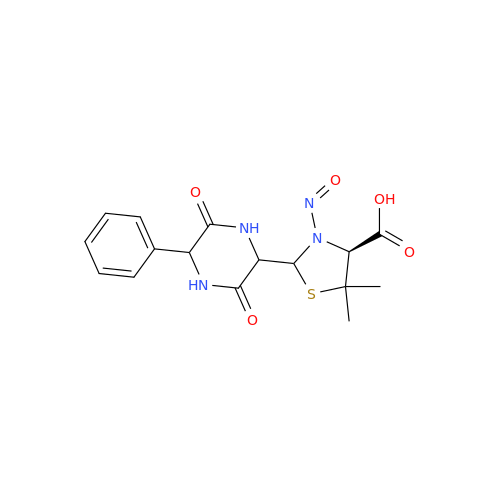
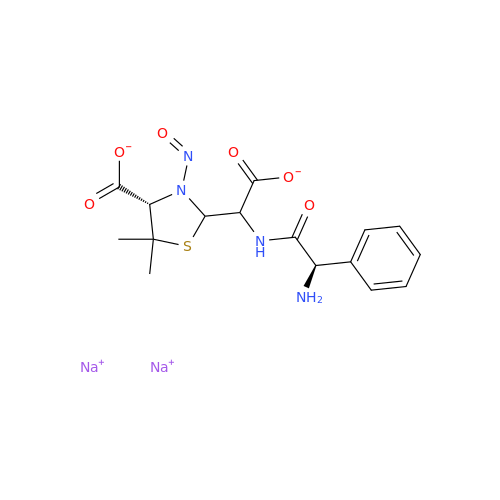
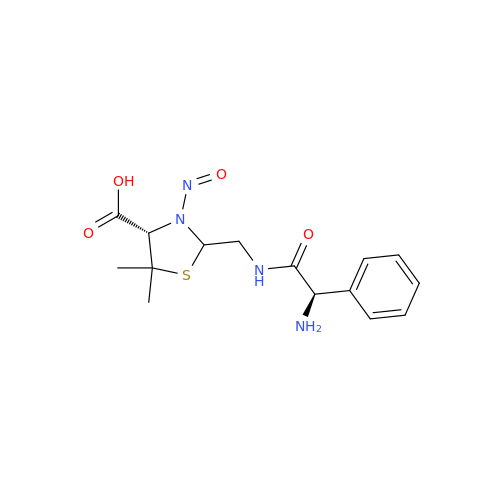
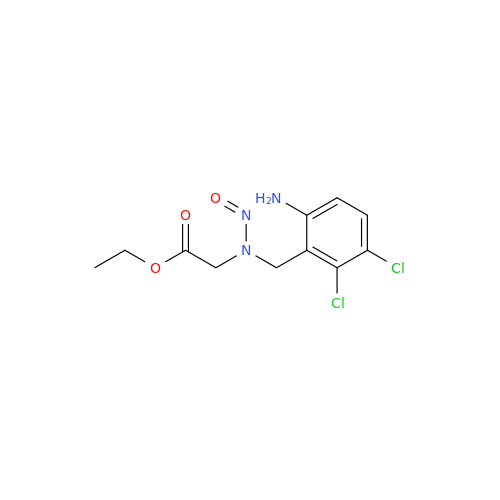
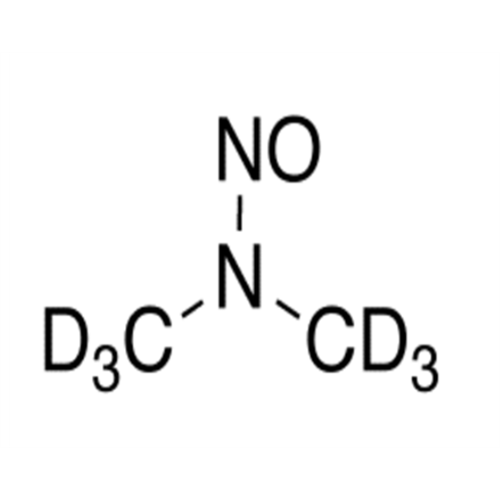
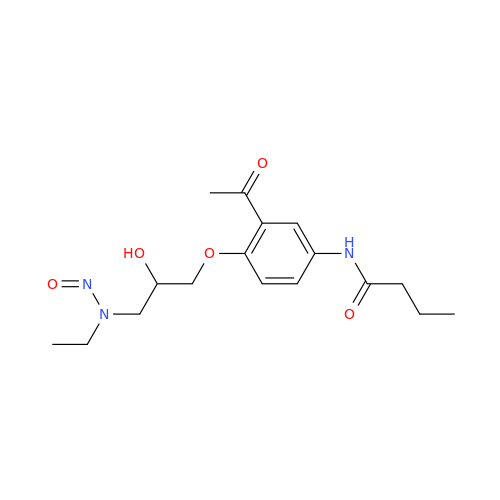
N-Nitroso Acebutolol EP Impurity I
Add to Cart
Bulk Enquiry
|
Chemical Name: N-Nitroso Acebutolol EP Impurity I
Synonym: N-Nitroso Acebutolol USP Related Compound I