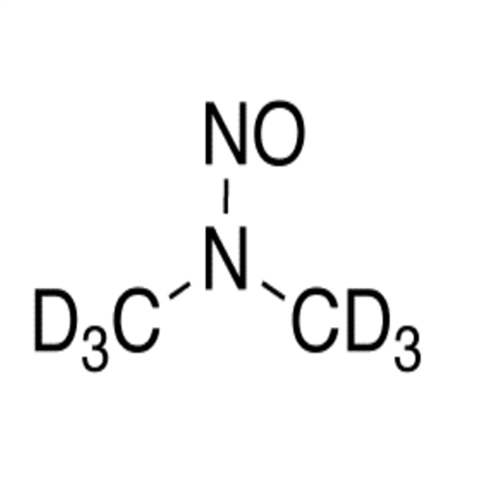
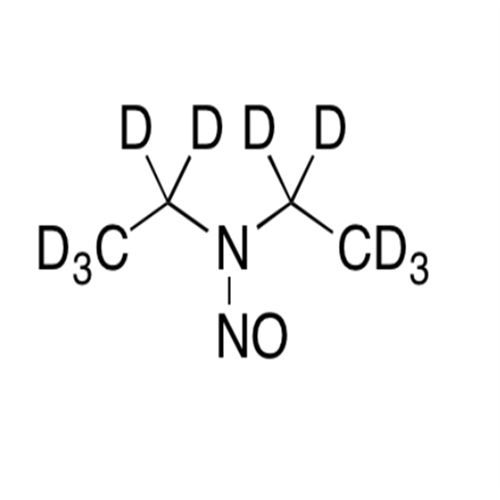
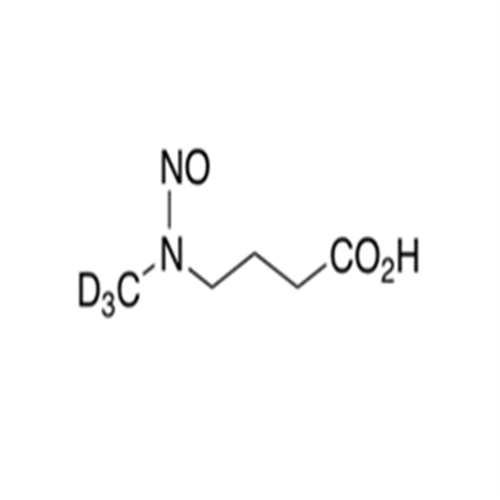
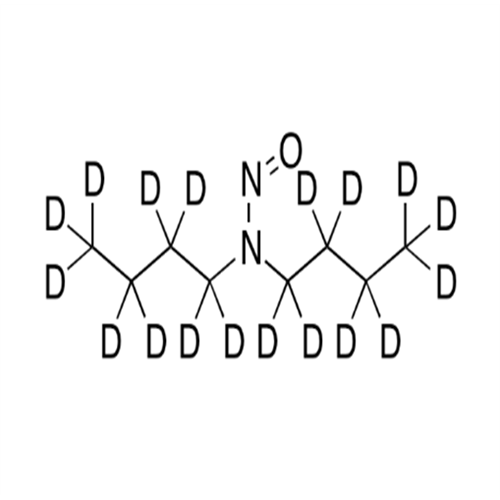
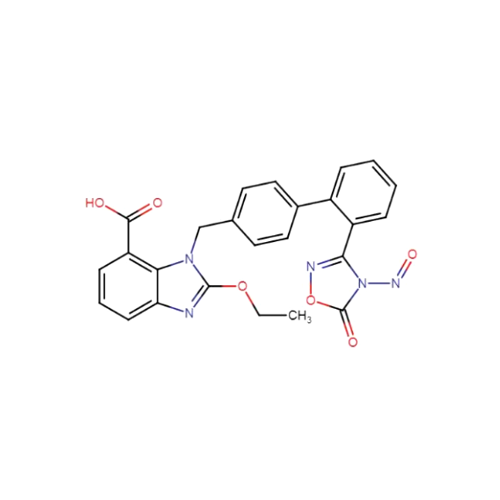
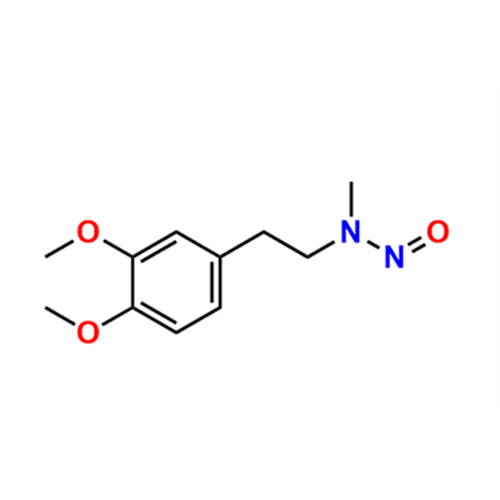
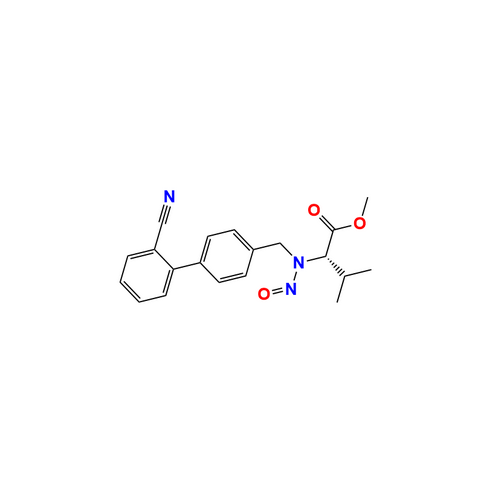
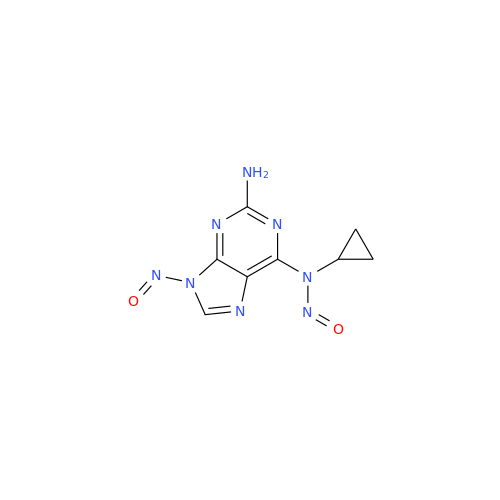
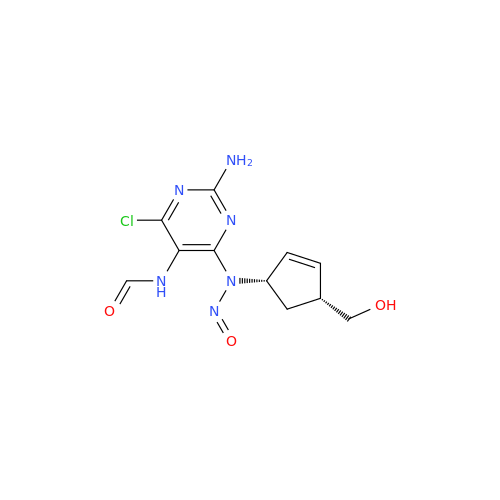
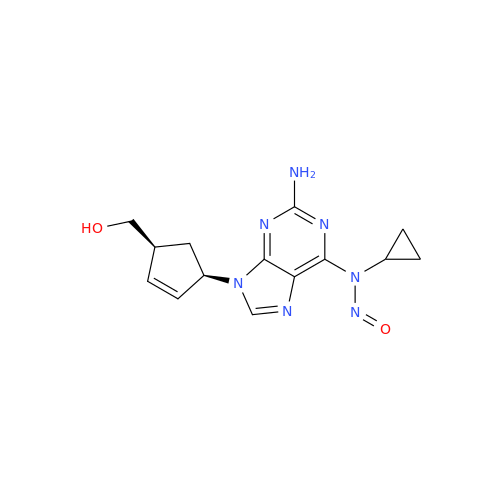
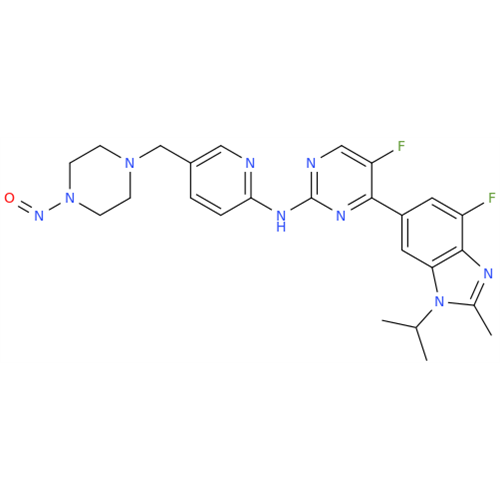
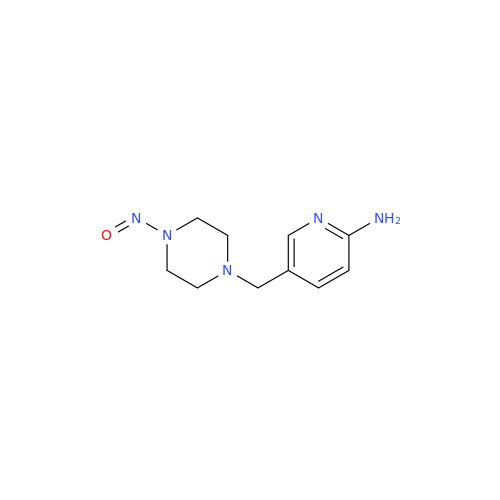
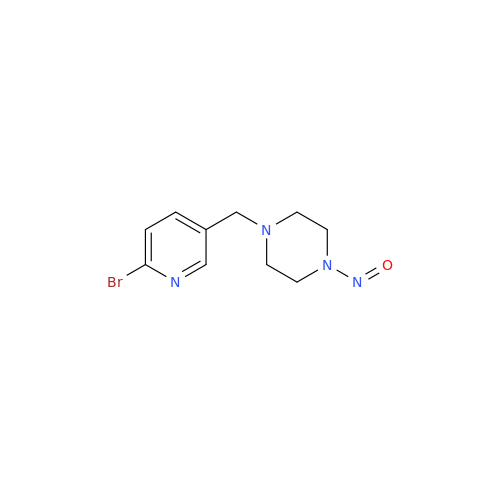
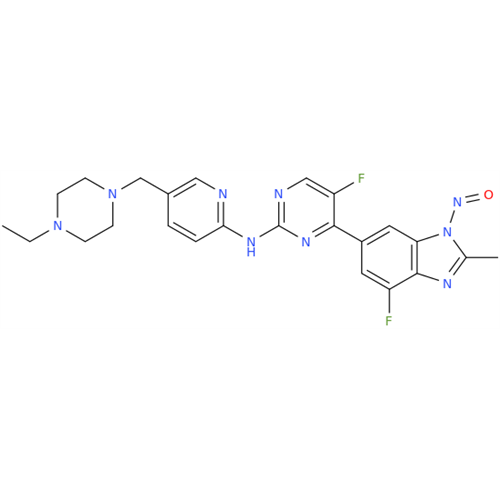
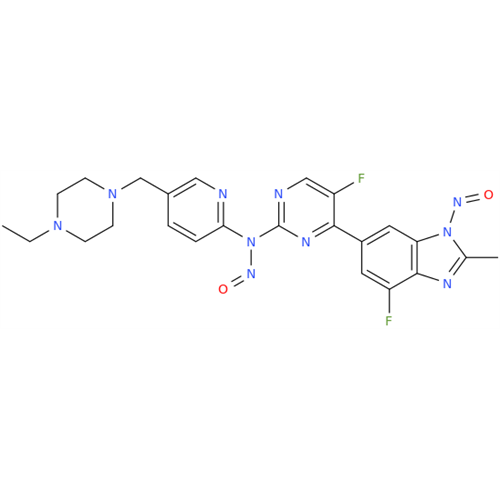
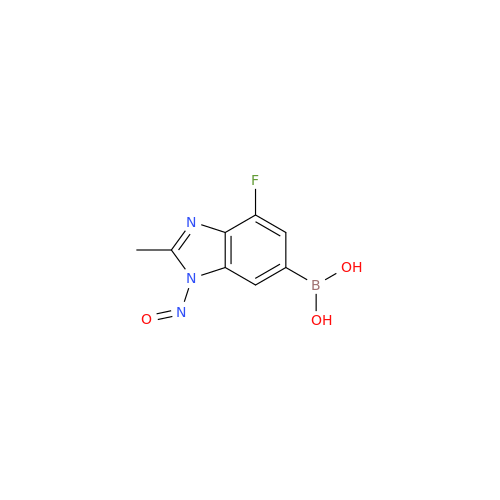
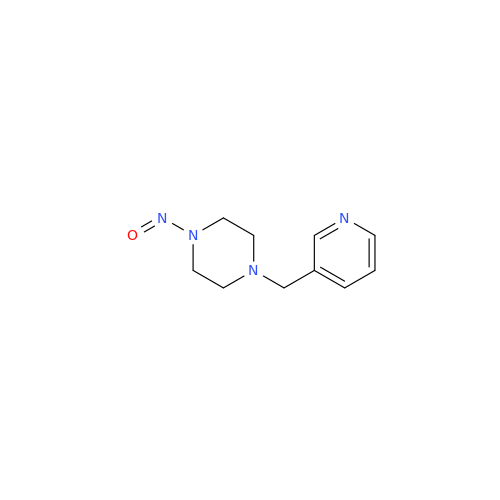
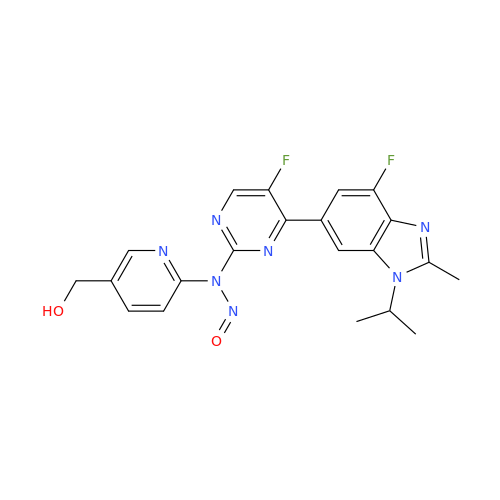
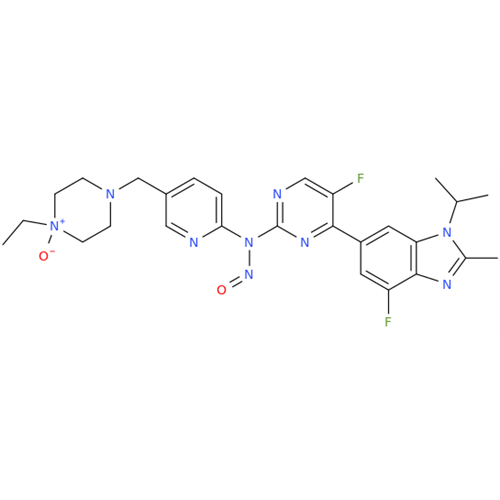
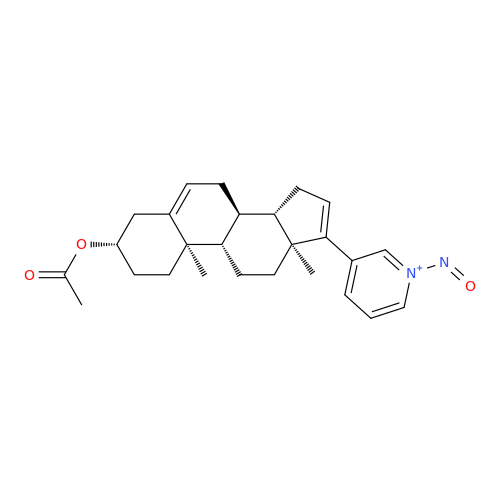
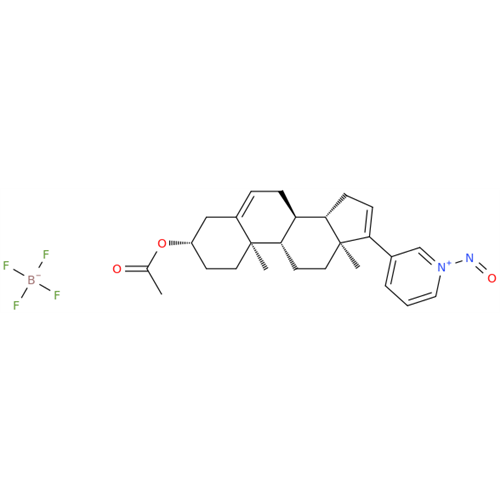
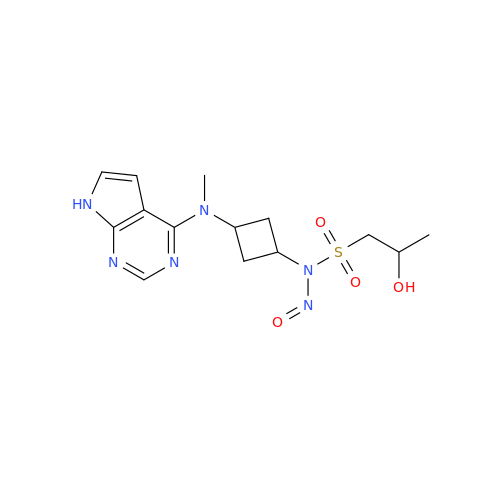
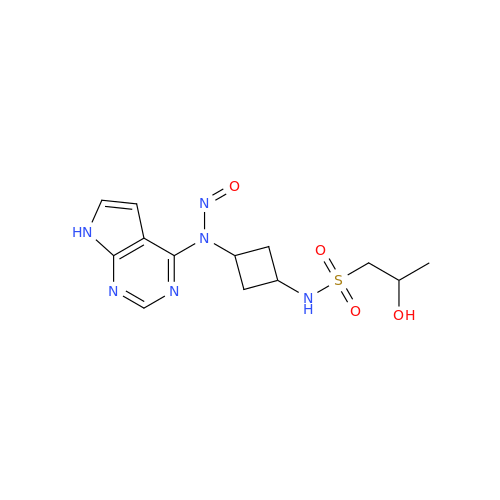
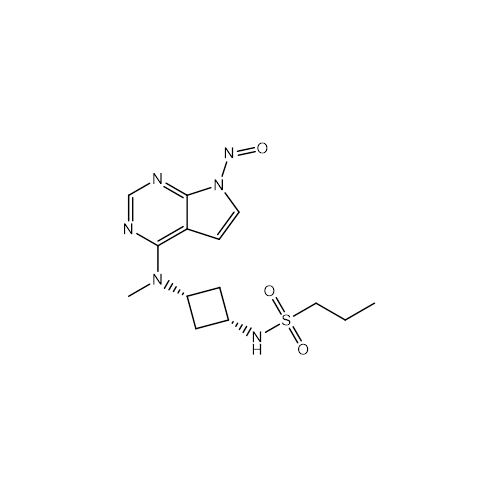
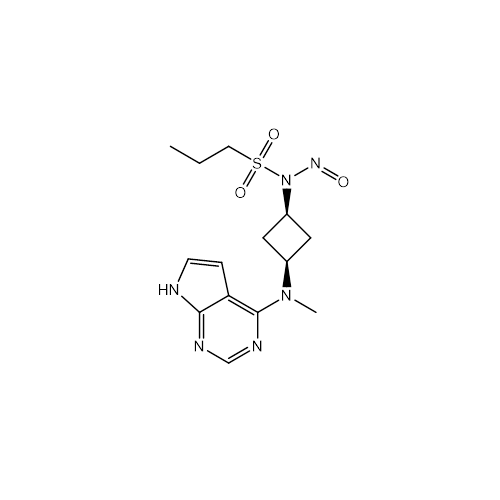
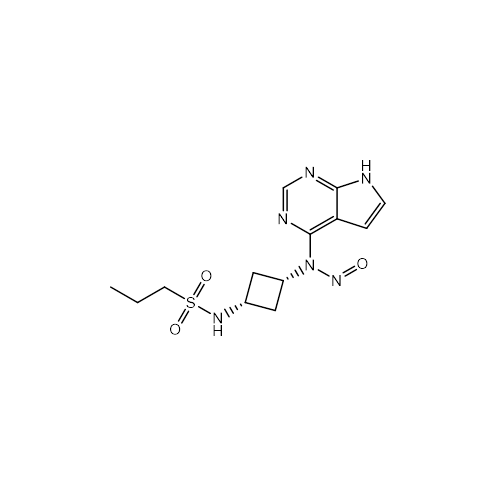
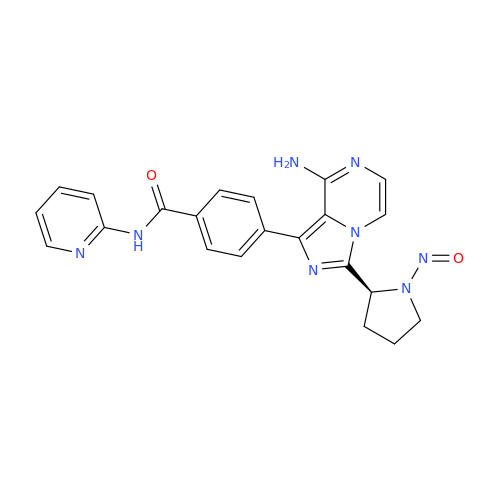
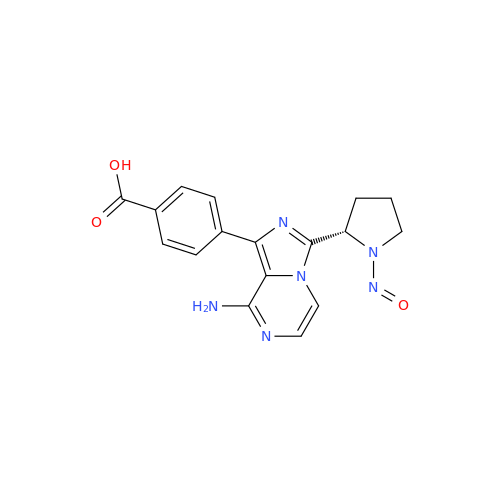
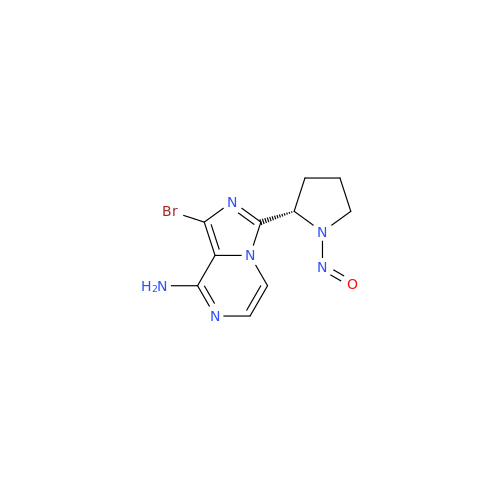
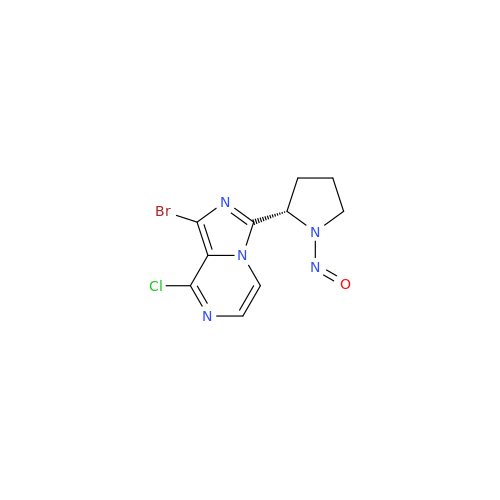
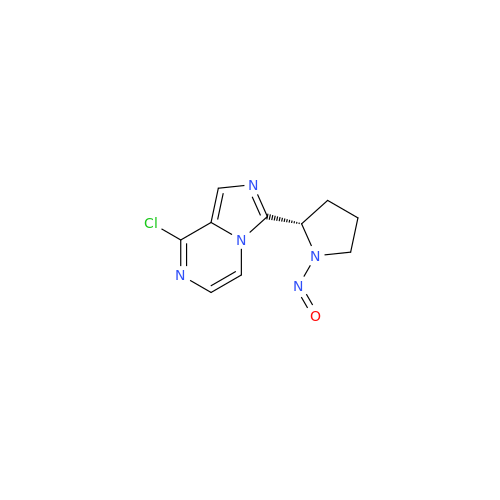
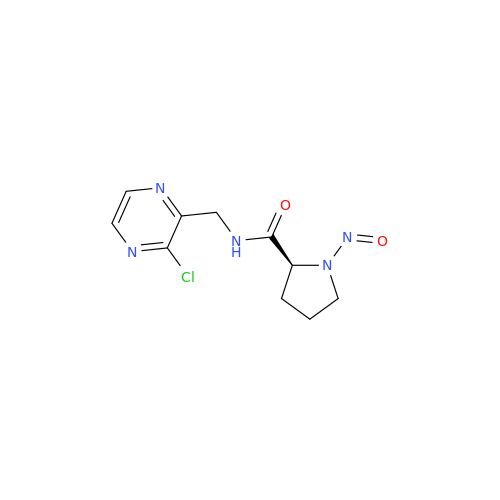
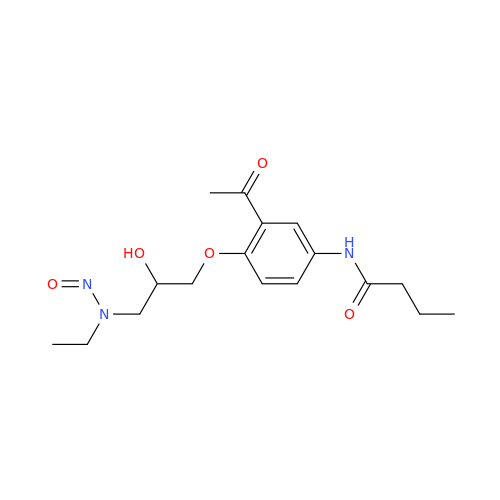
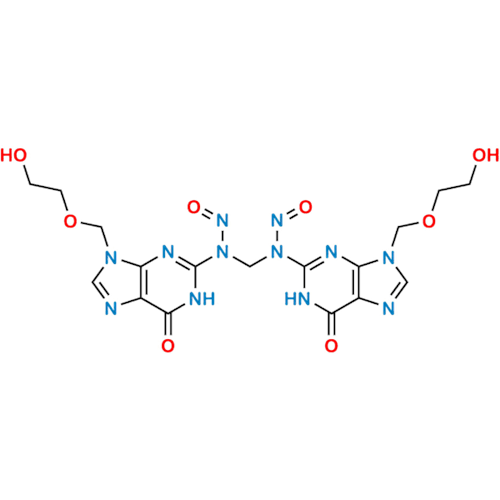
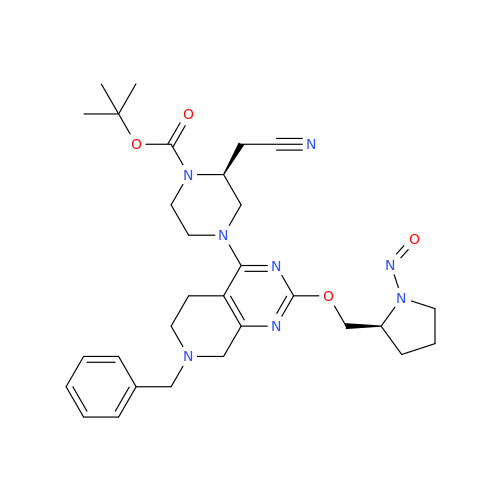
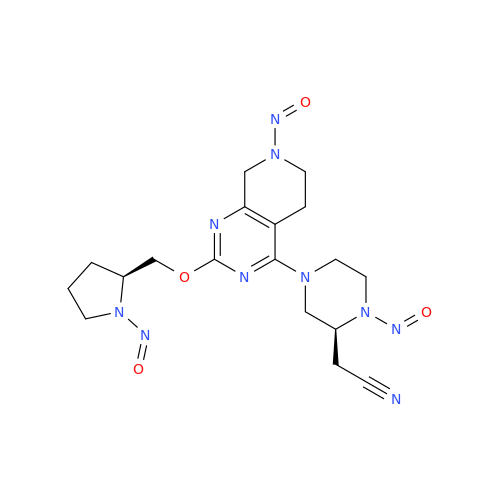
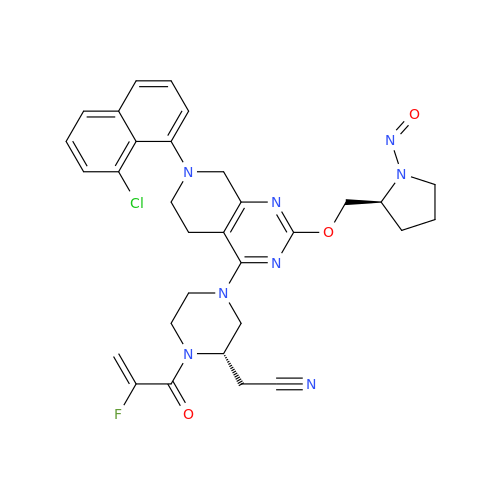
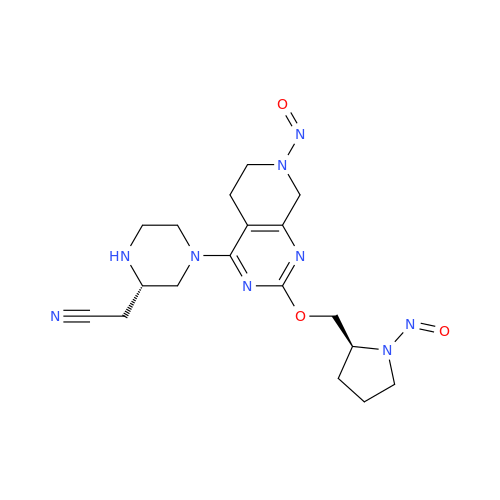
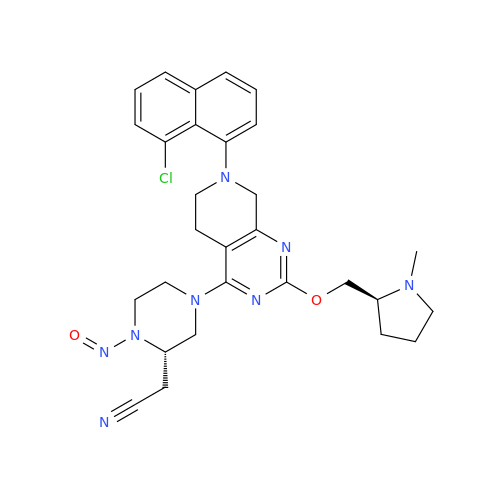
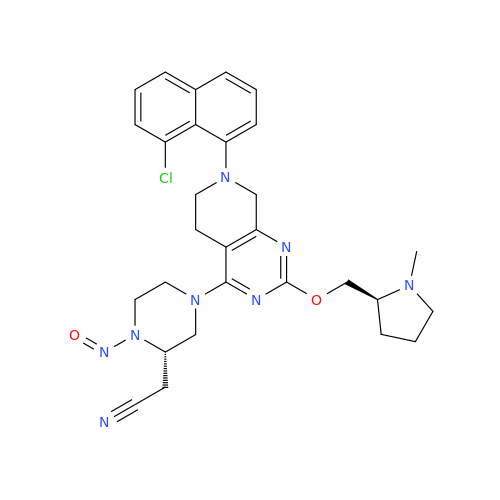
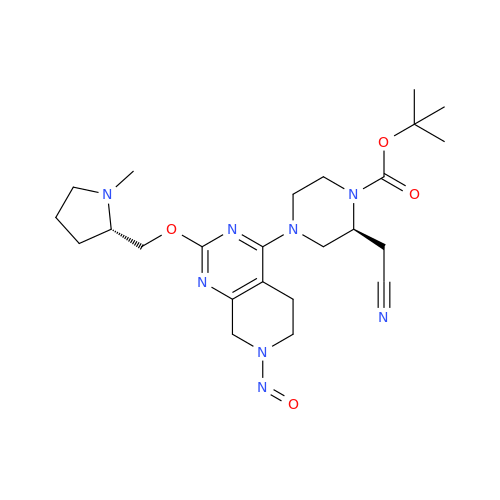
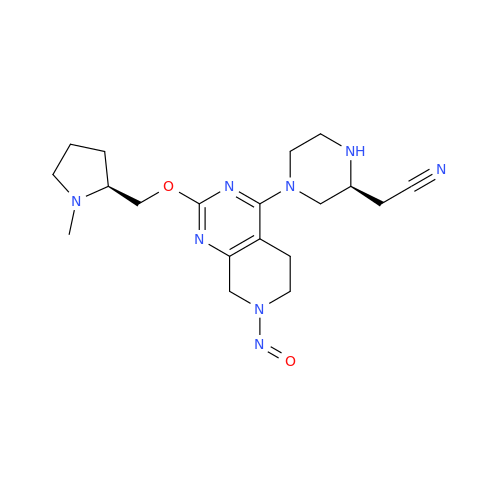
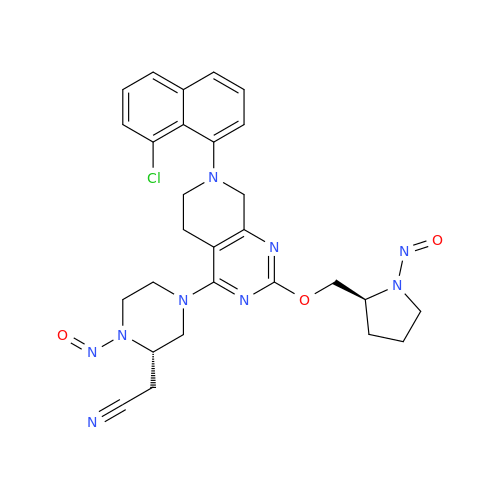
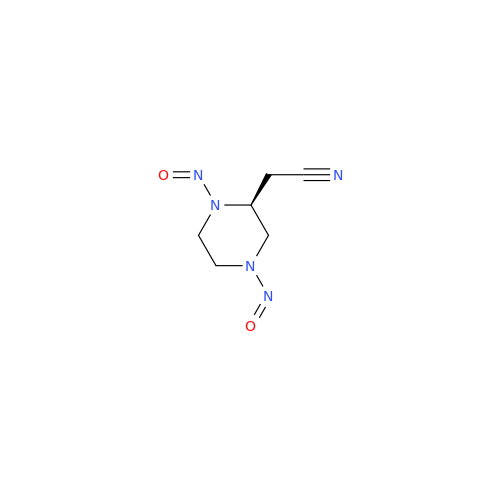
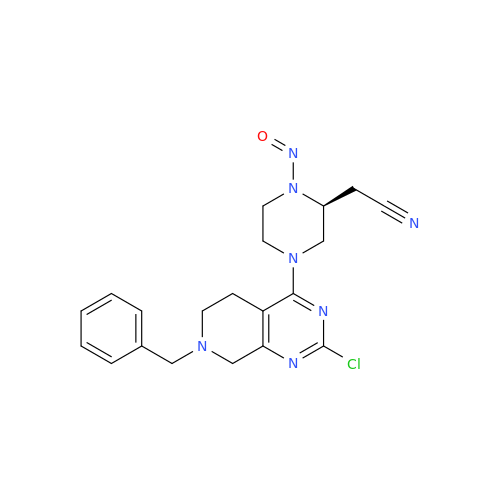
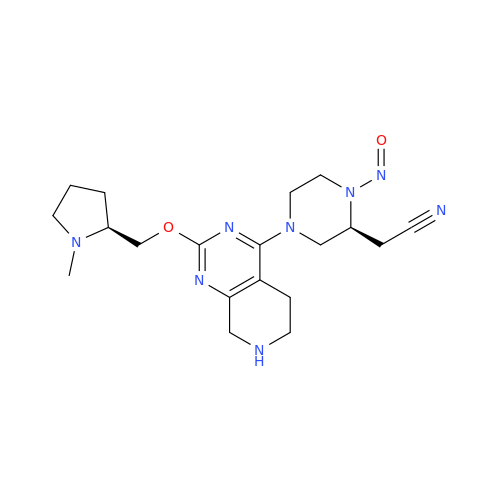
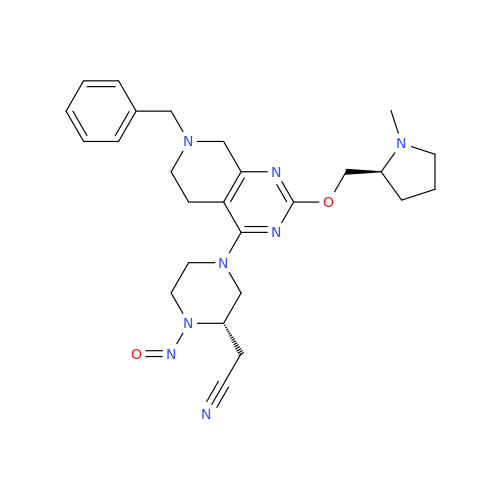
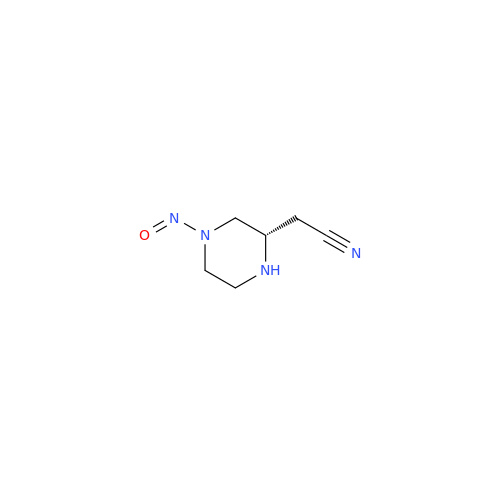
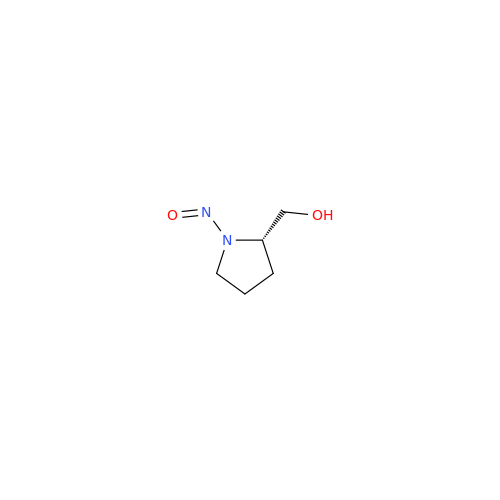
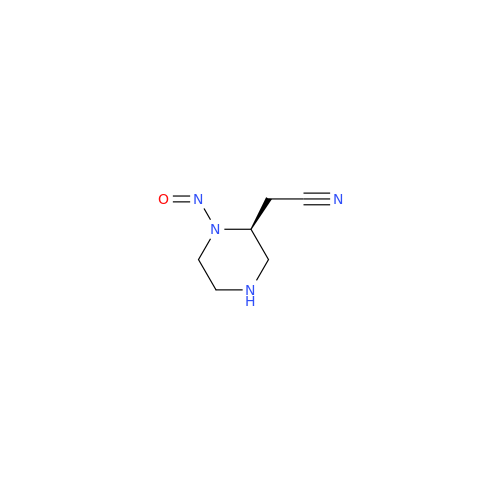
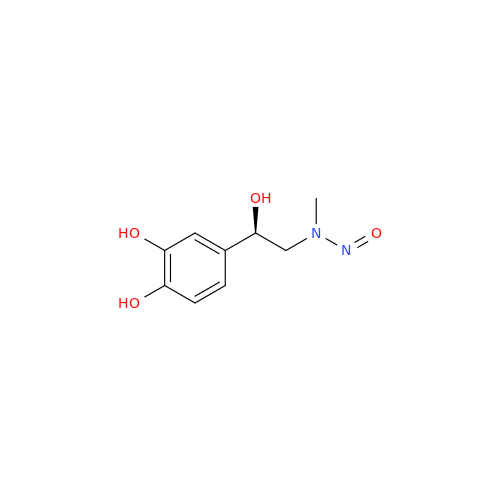
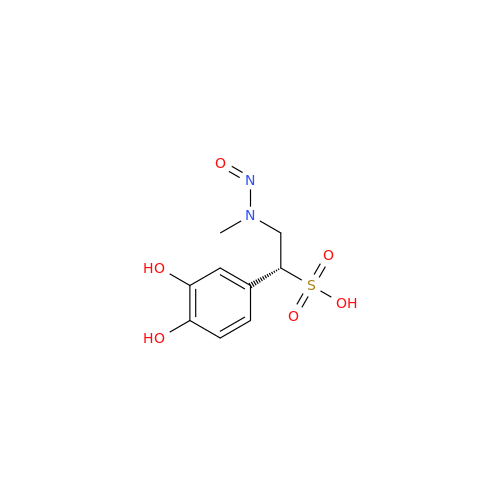
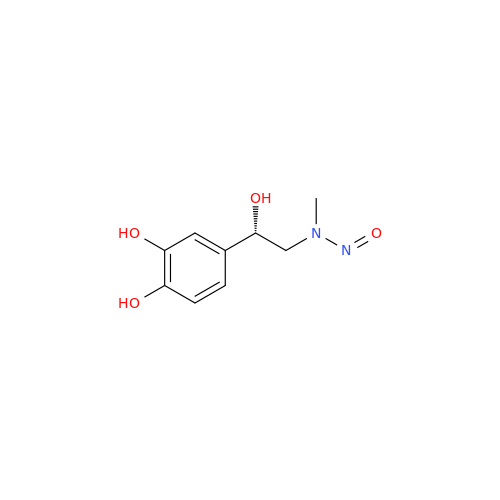
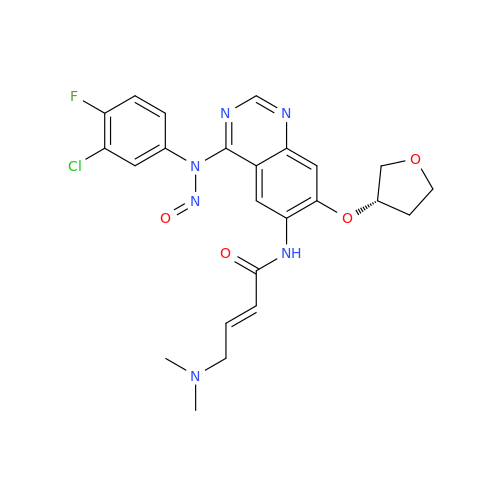
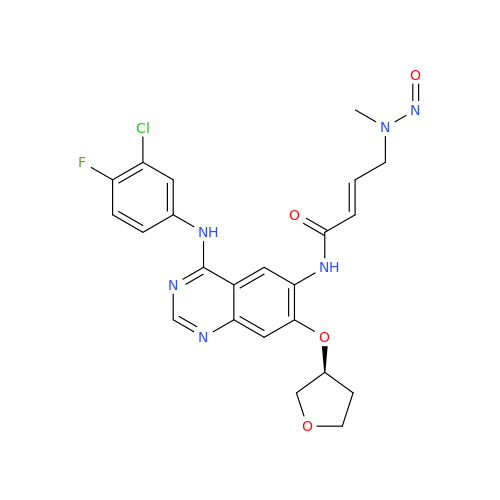
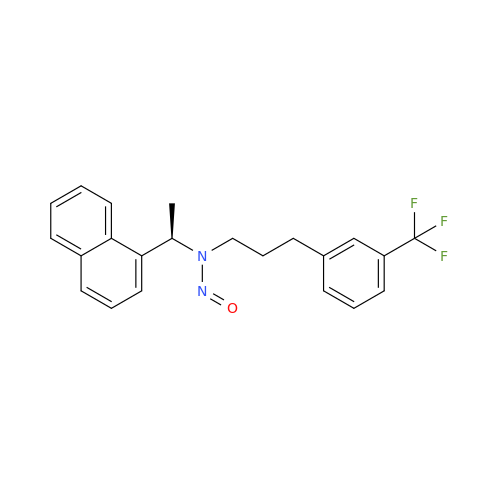
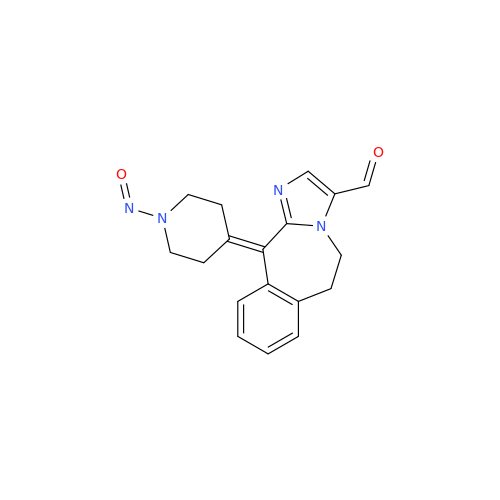
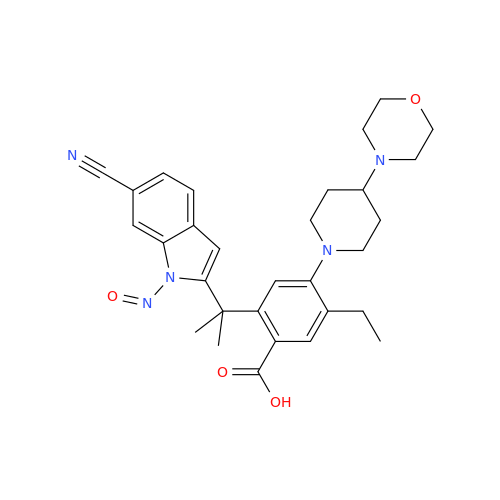
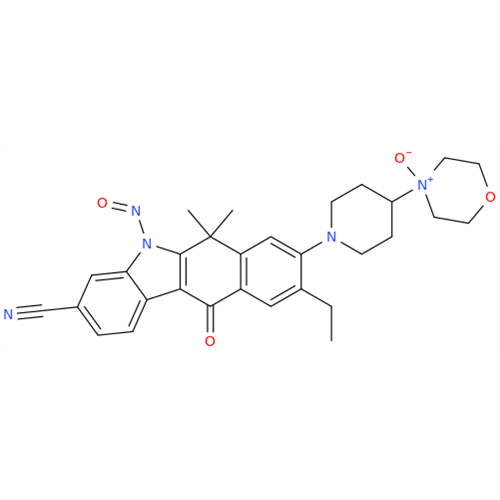
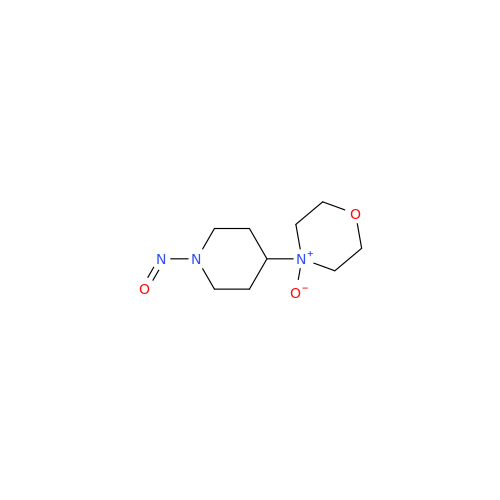
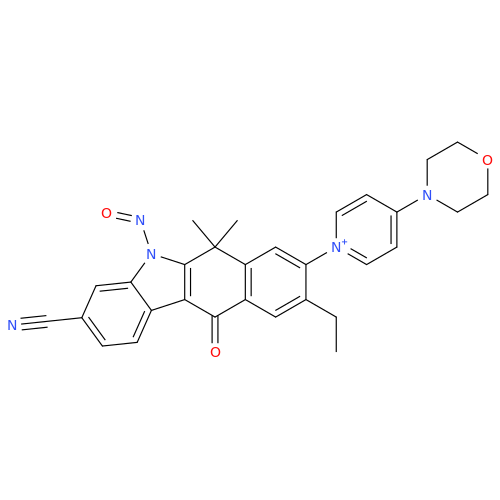
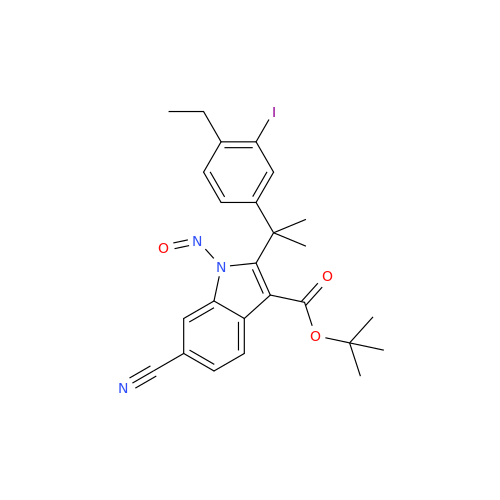
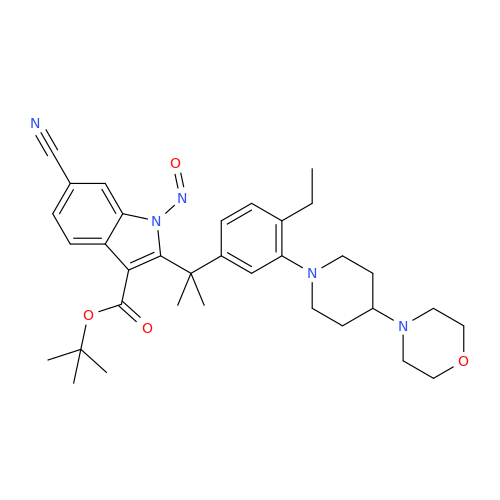
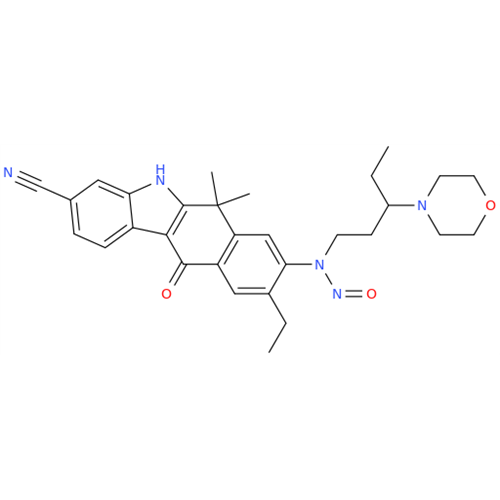
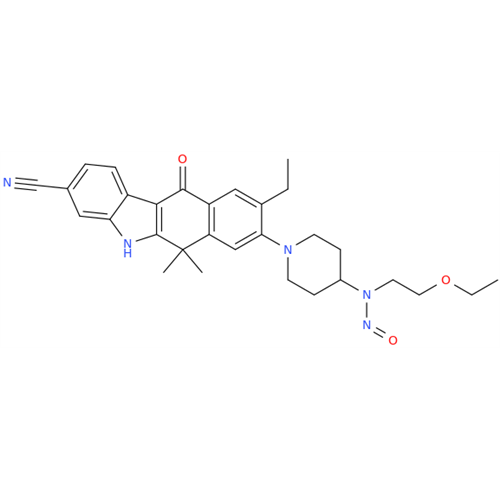
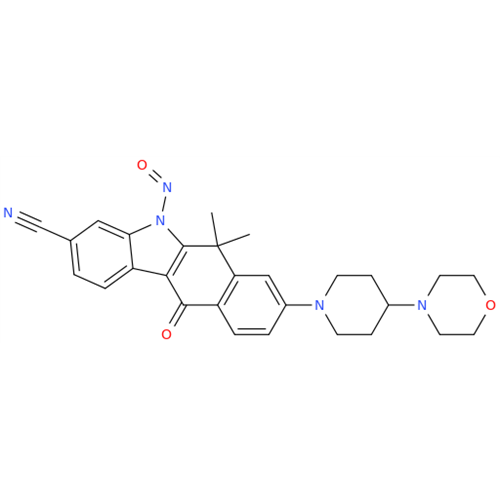
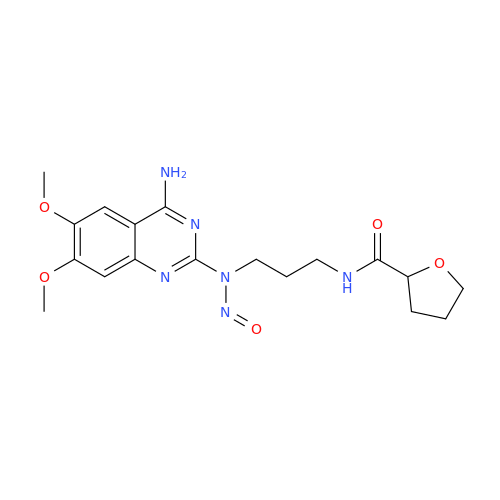
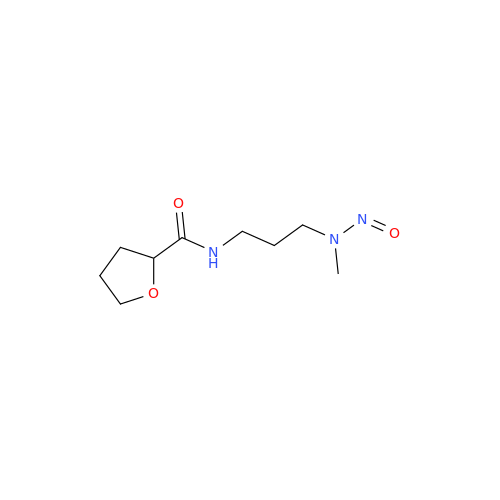
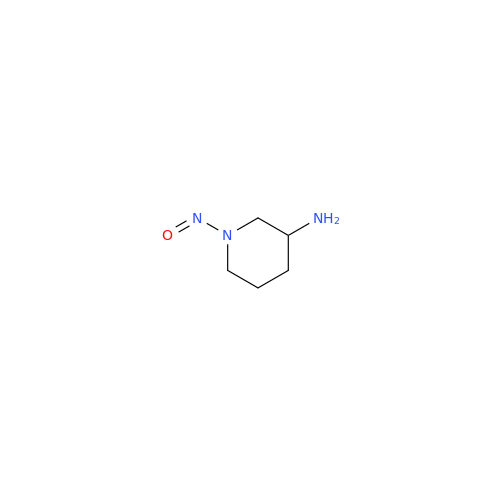
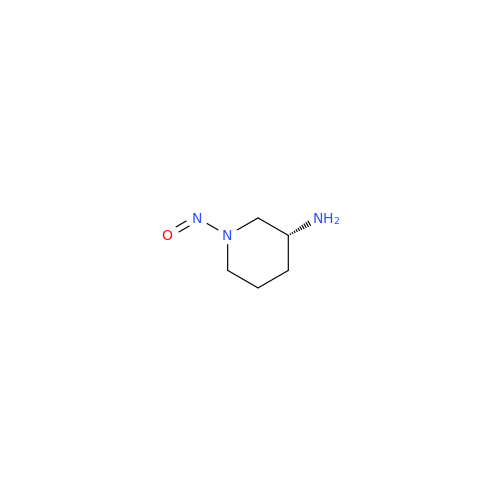
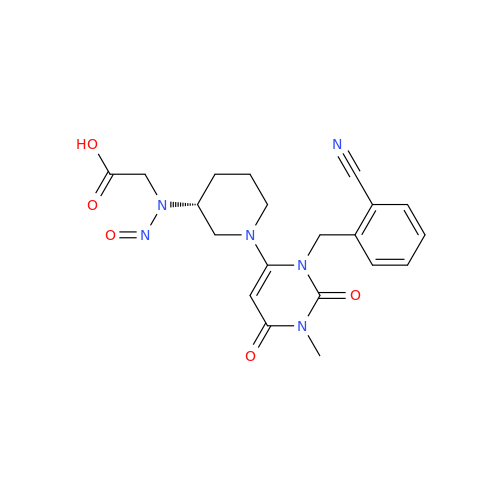
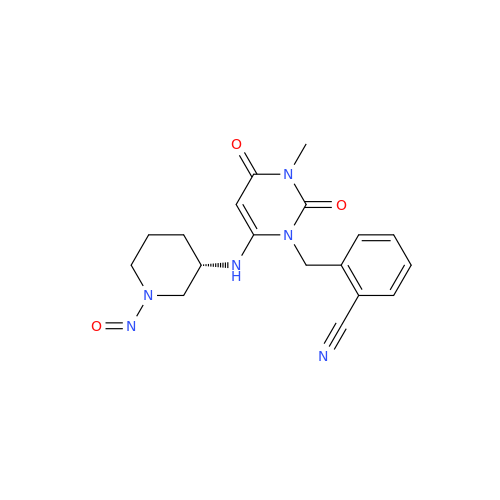
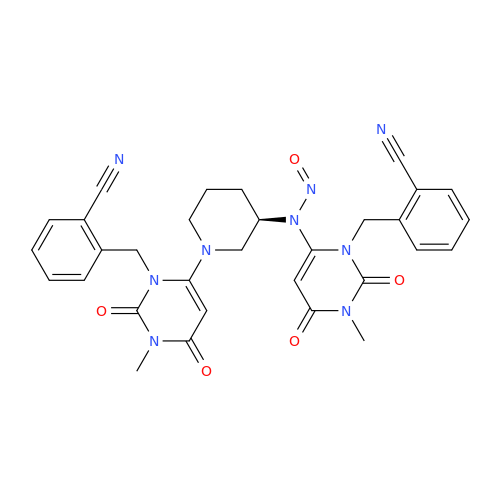
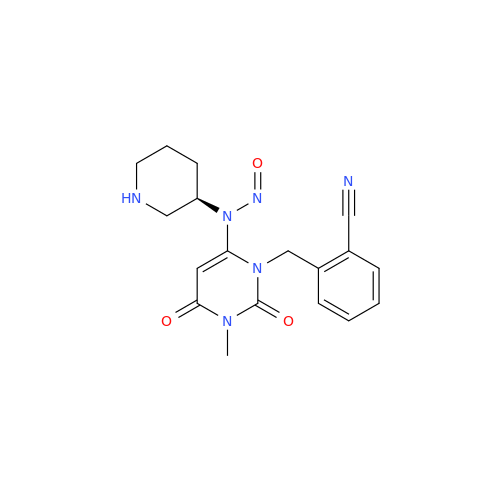
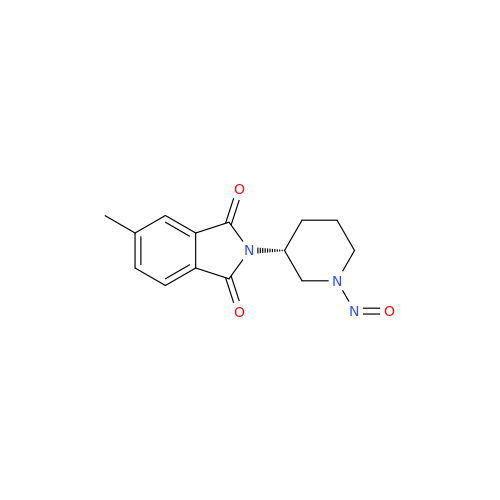
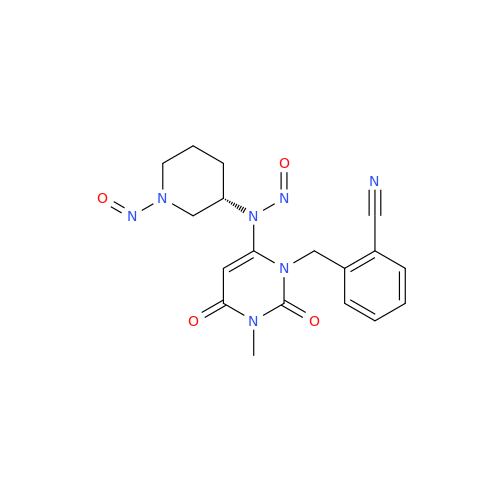
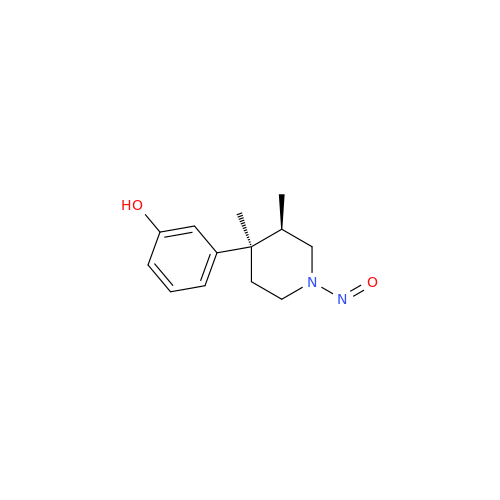
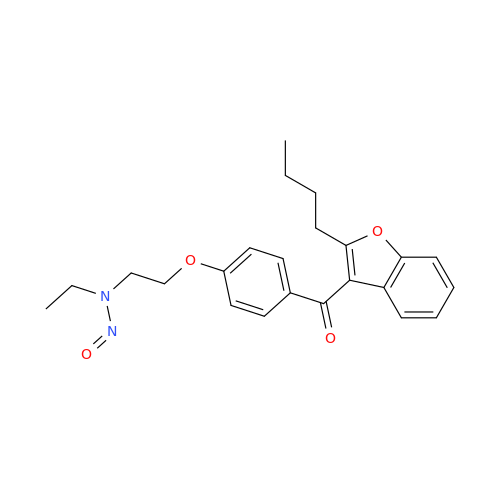
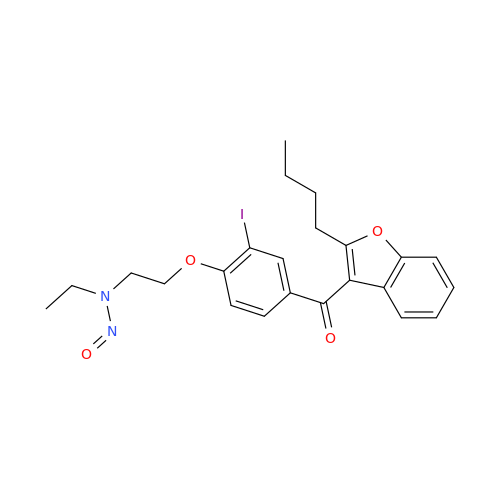
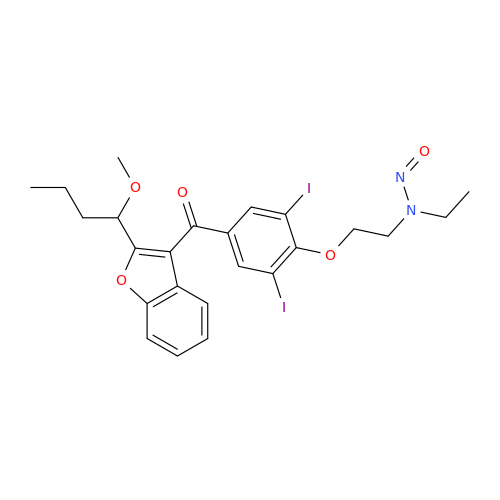
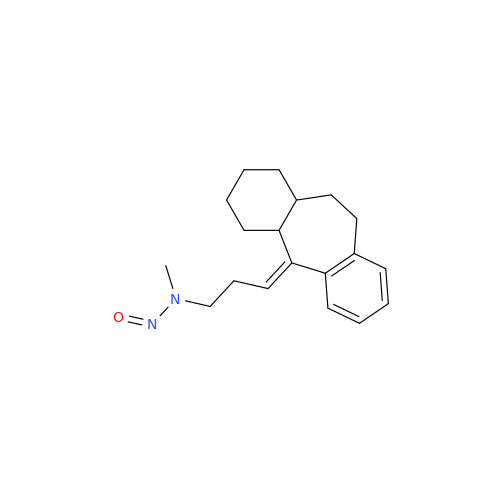
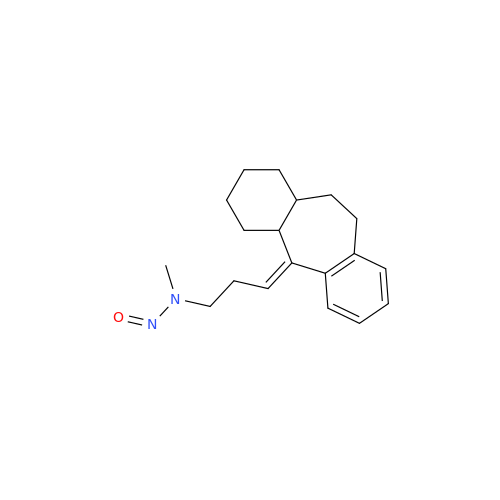
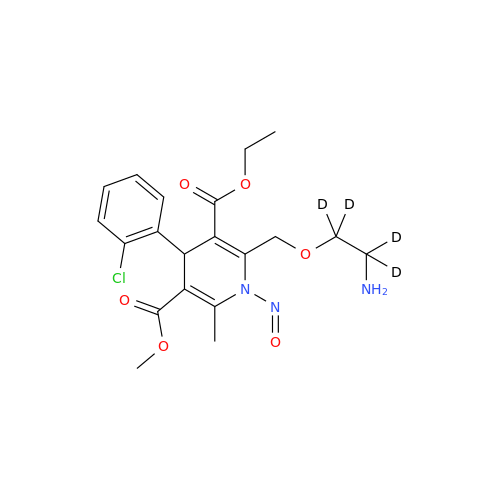
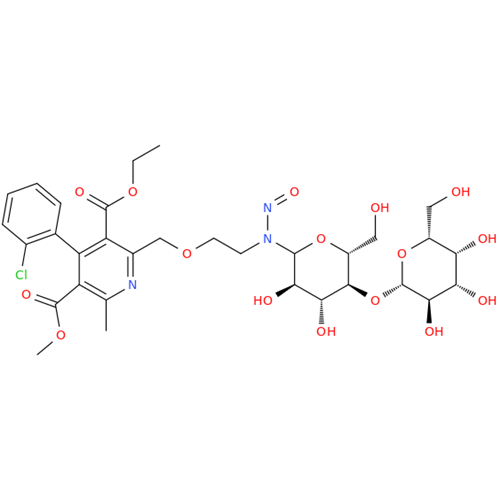
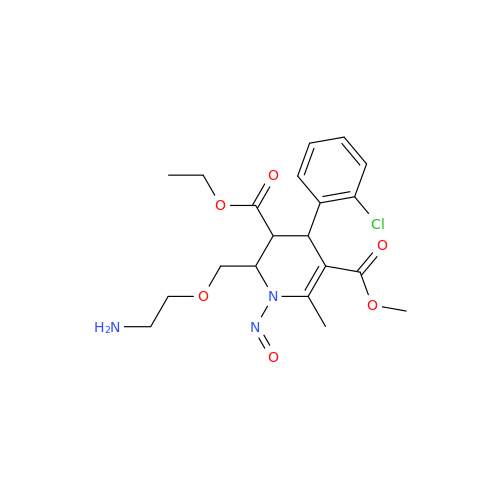
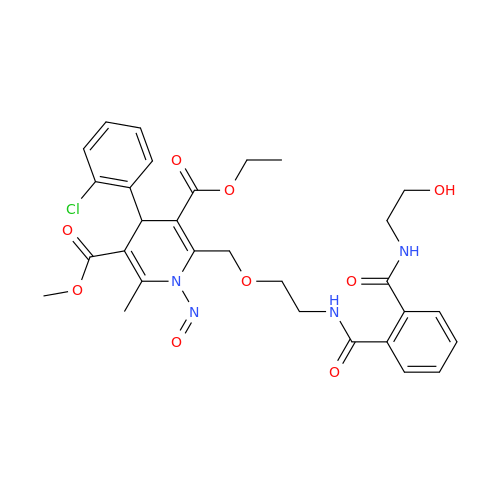
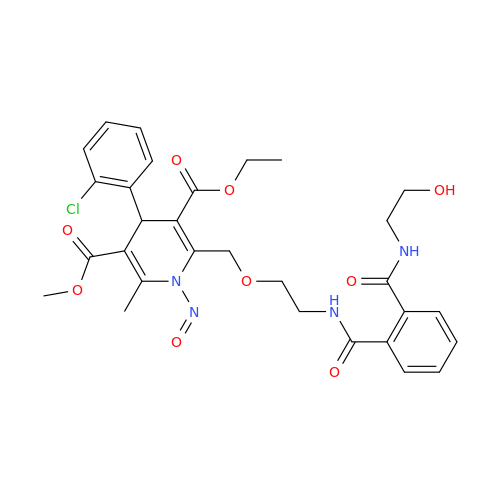
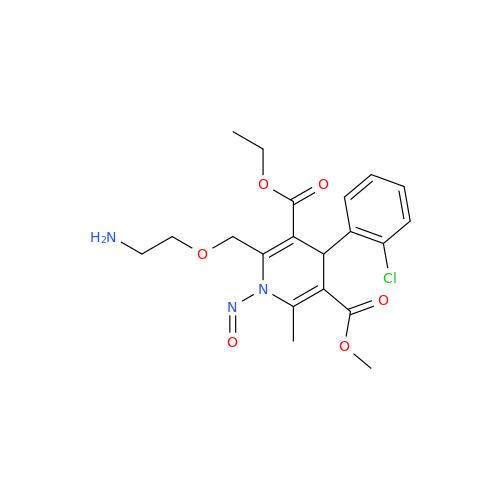
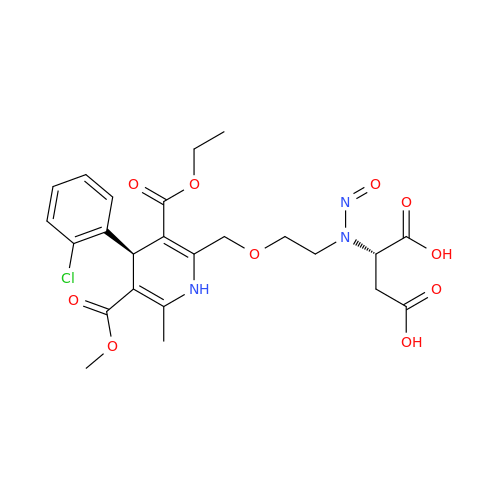
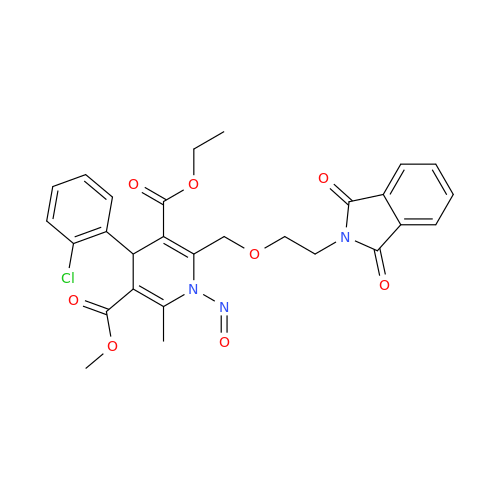
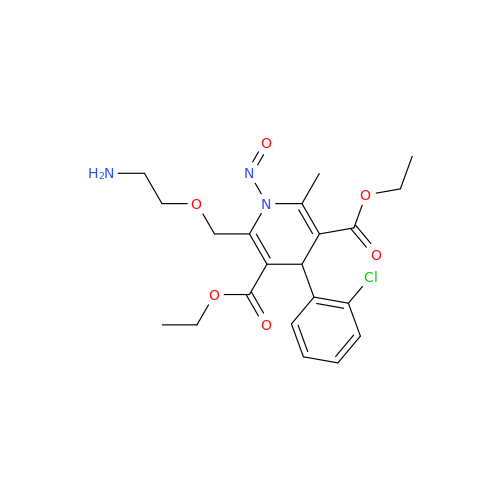
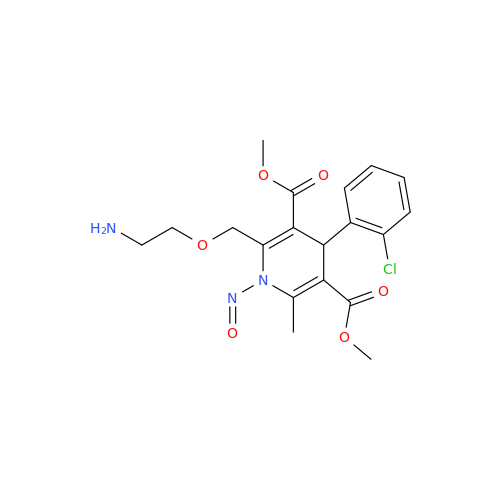
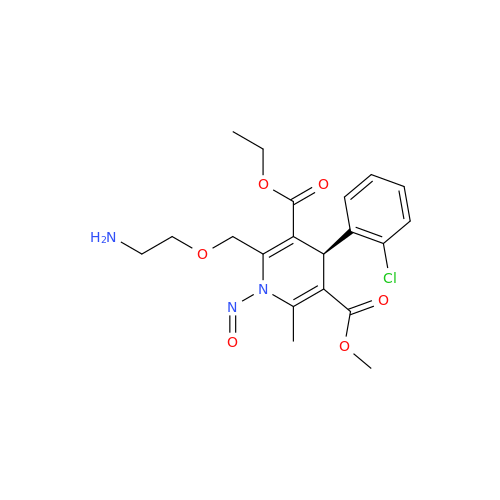
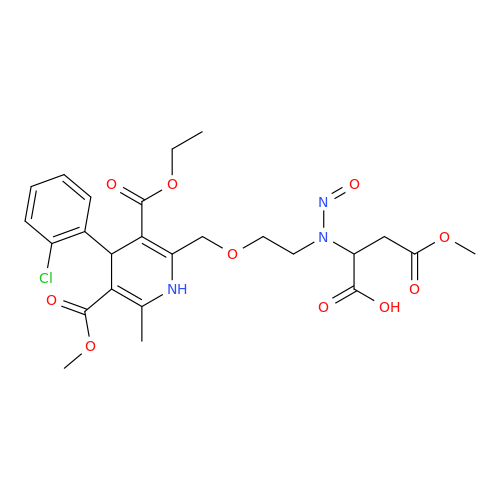
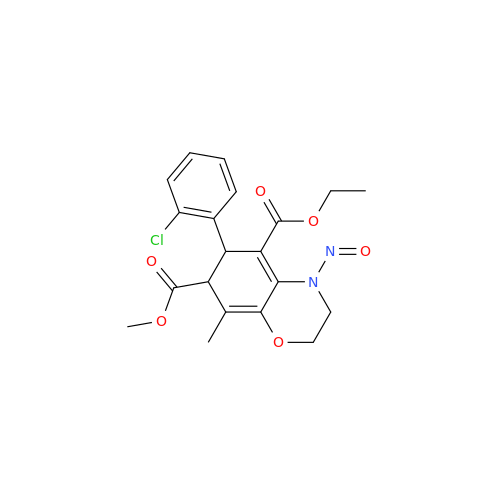
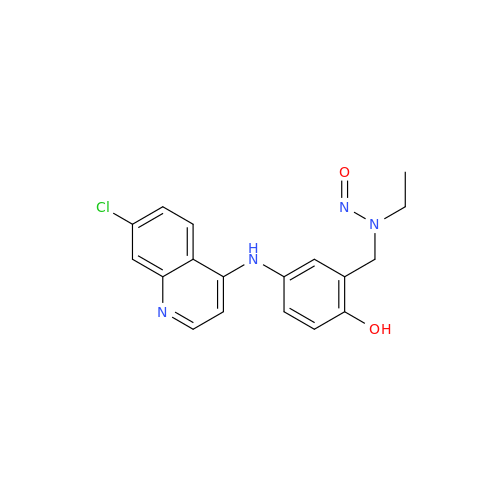
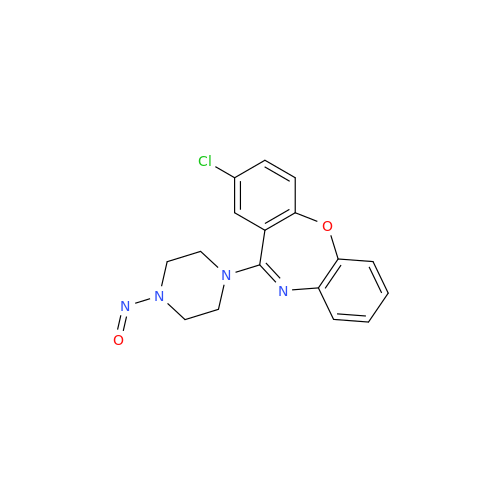
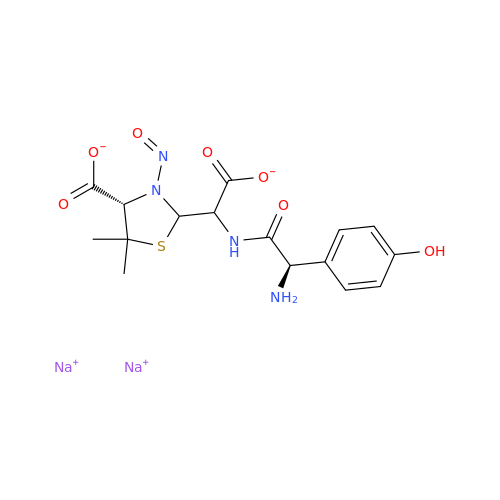
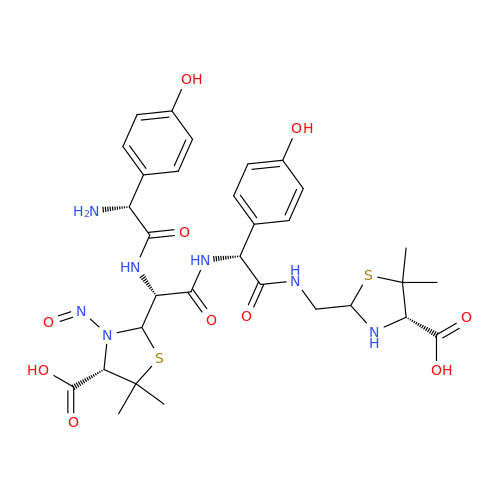
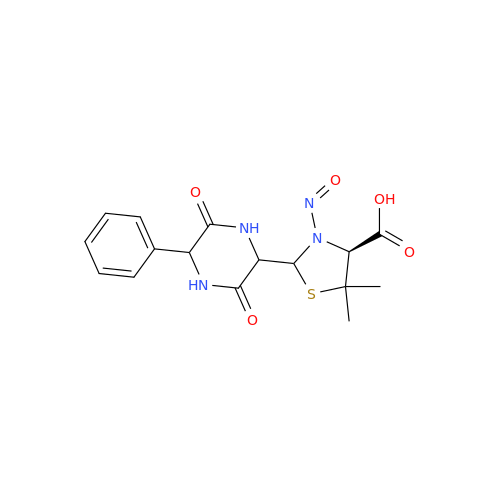
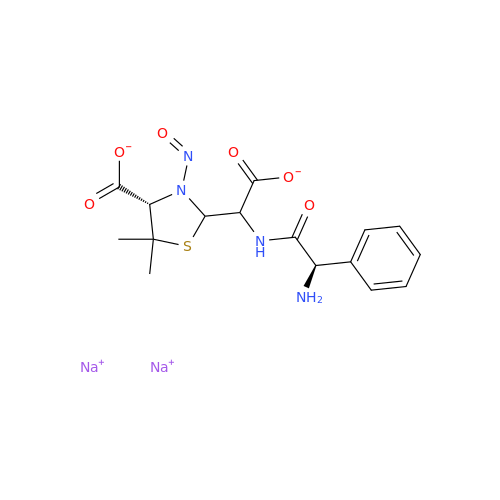
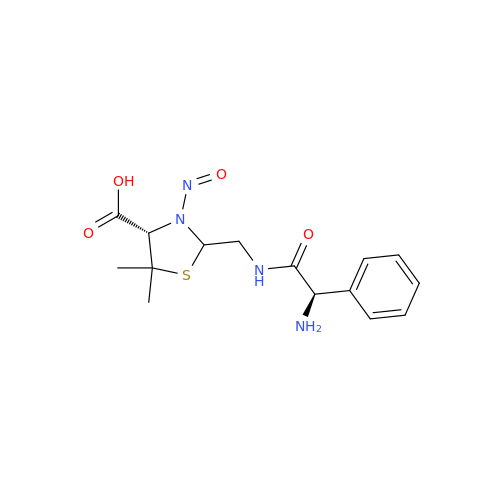
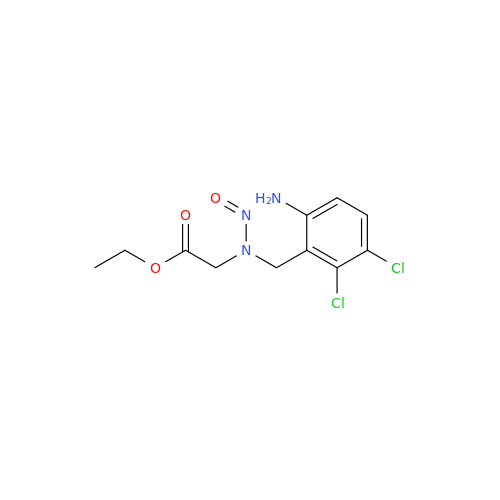
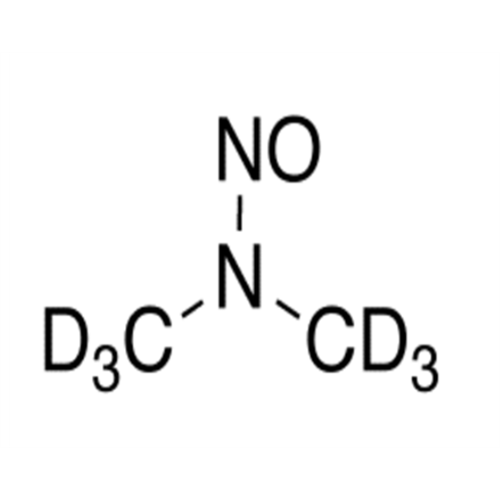
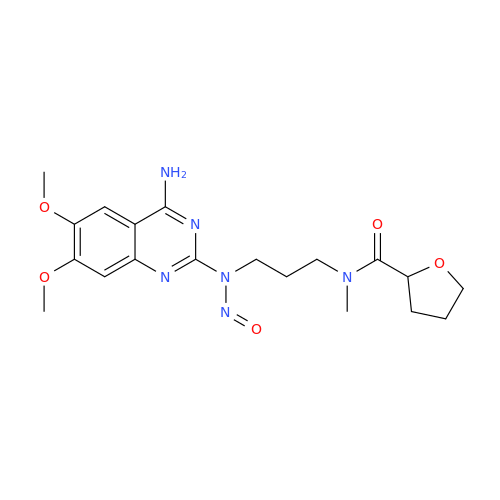
N-Nitroso Alfuzosin EP Impurity C
Add to Cart
Bulk Enquiry
|
Chemical Name: N-Nitroso Alfuzosin EP Impurity C
Synonym: N-(3-((4-Amino-6,7-dimethoxyquinazolin-2-yl)(nitroso)amino)propyl)-N-methyltetrahydrofuran-2-carboxamide