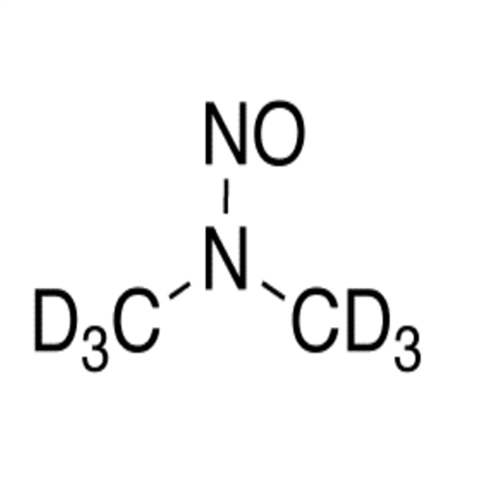
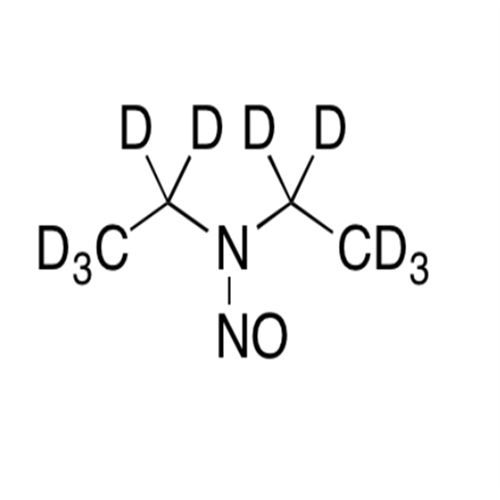
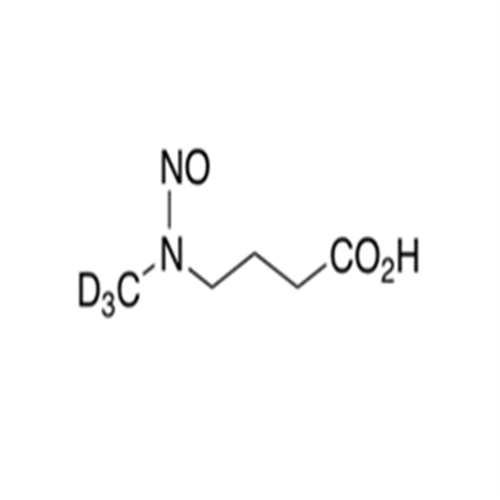
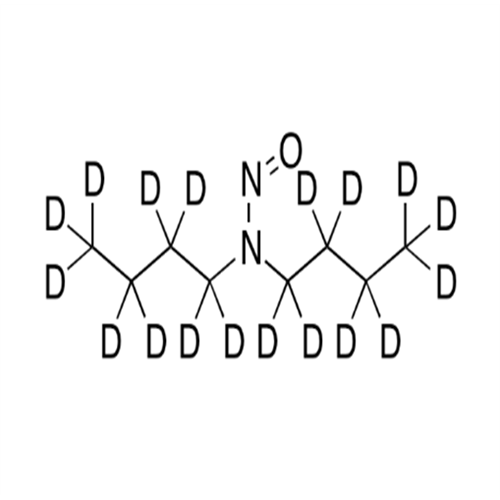
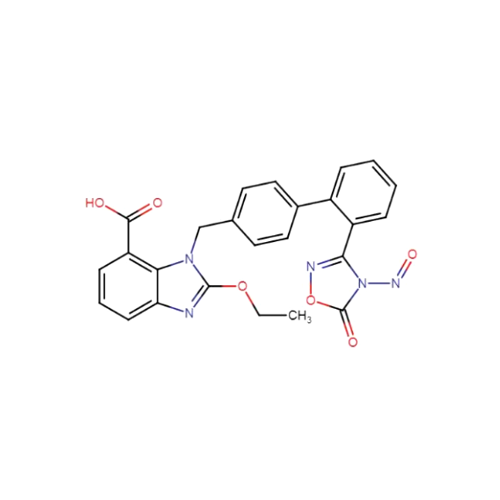
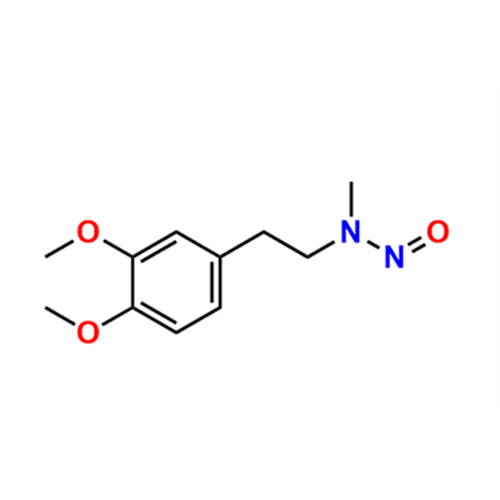
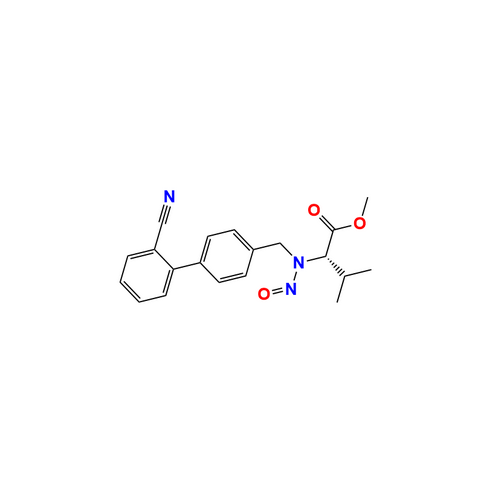
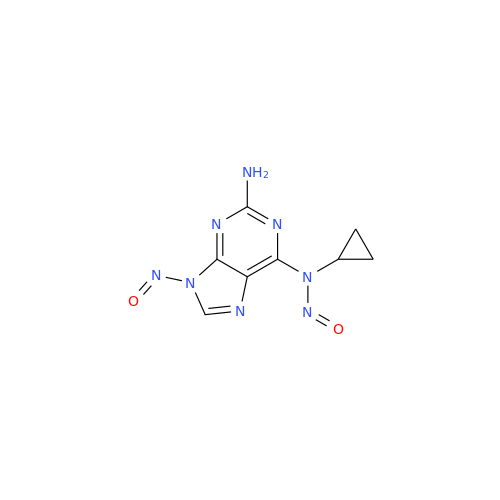
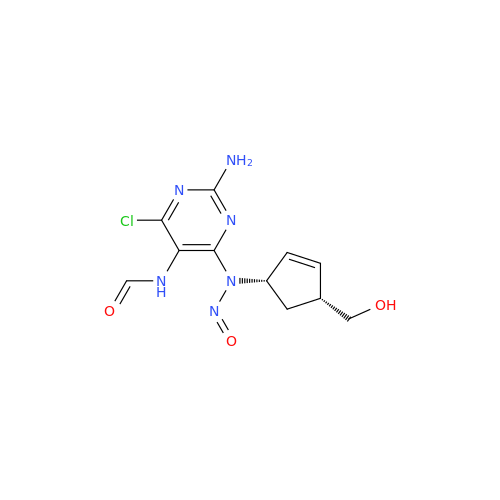
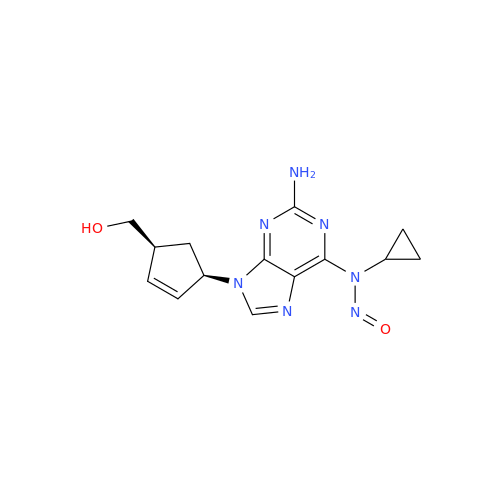
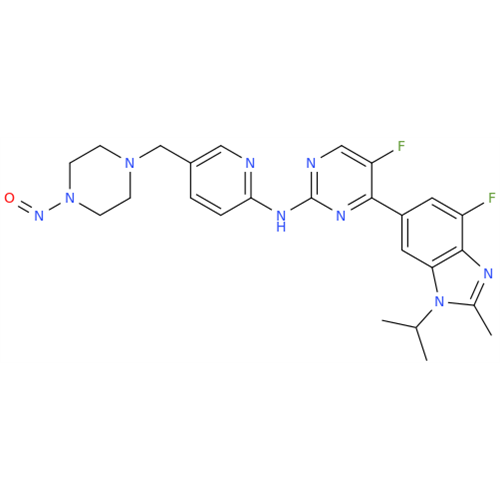
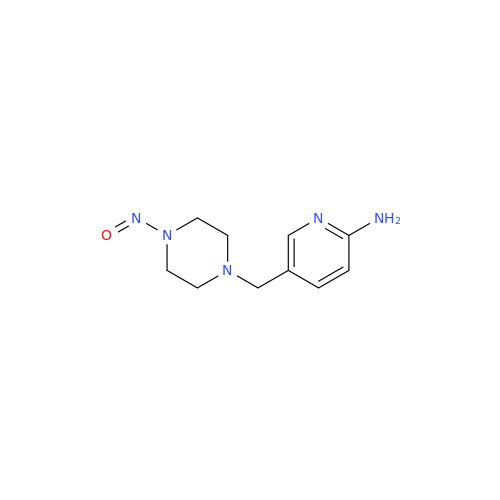
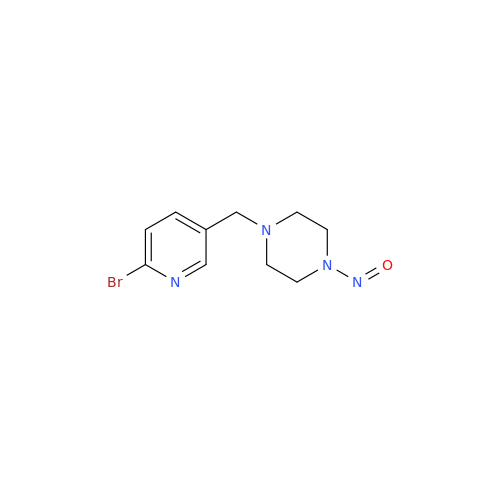
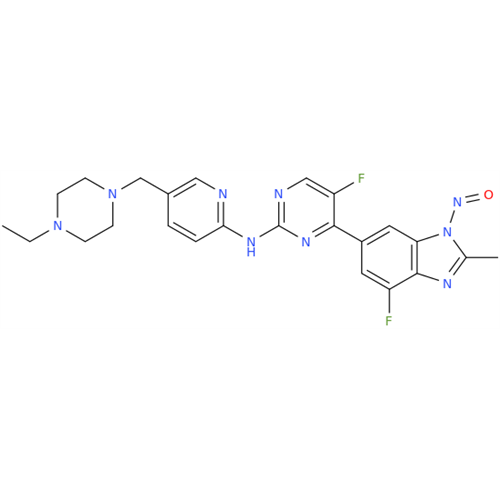
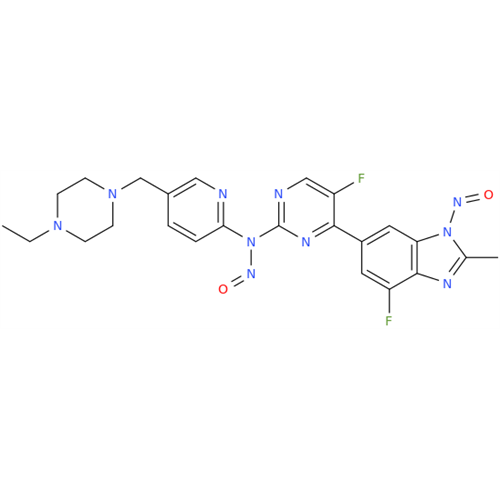
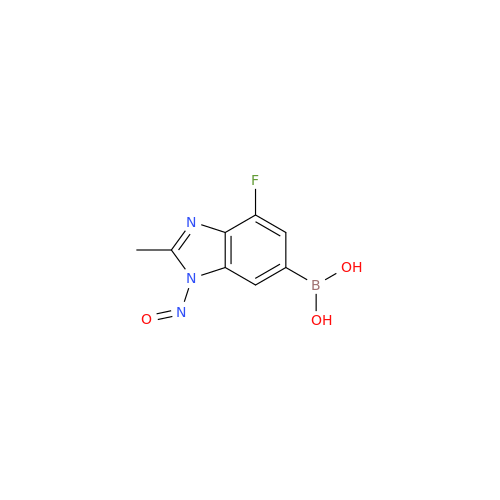
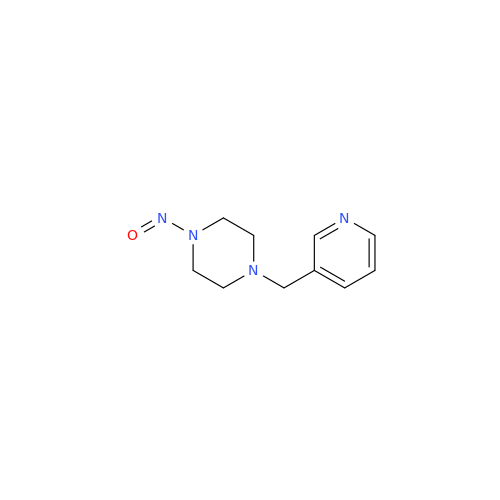
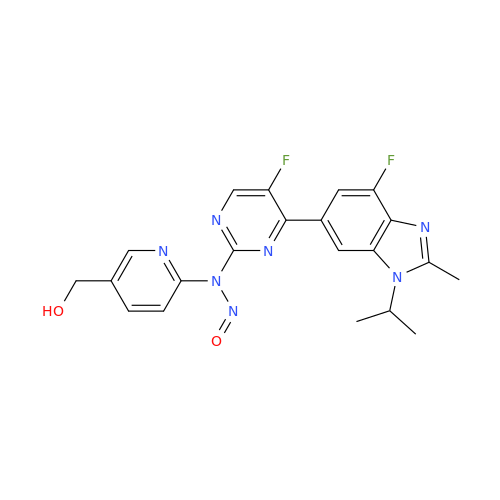
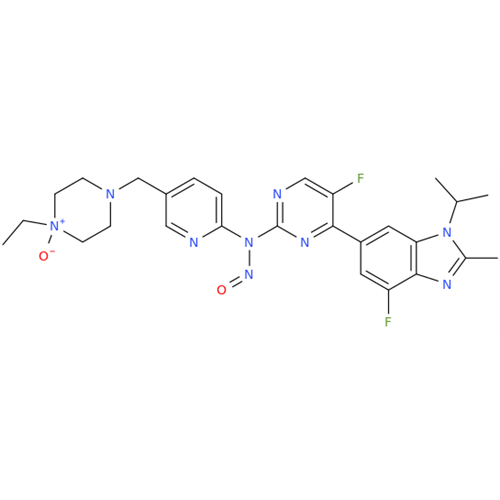
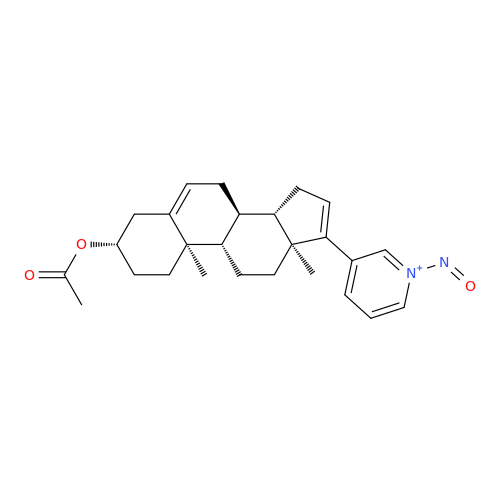
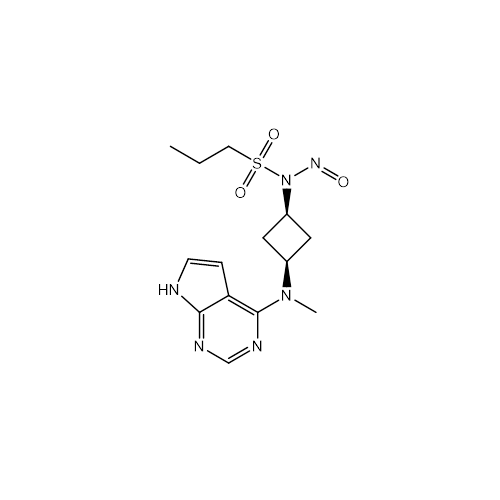
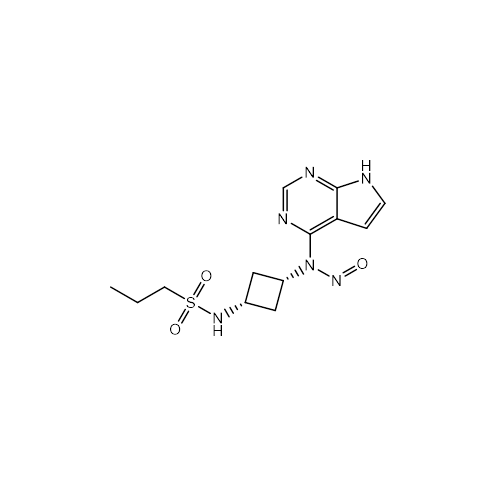
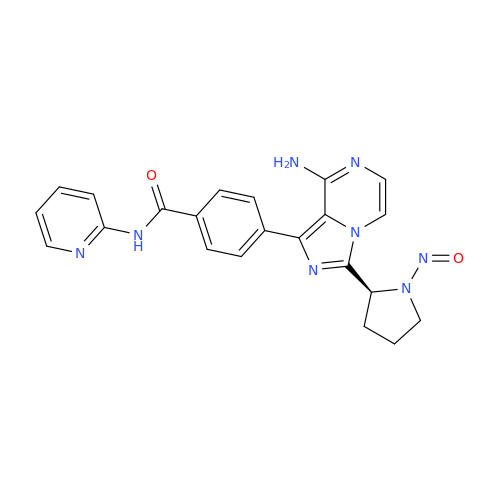
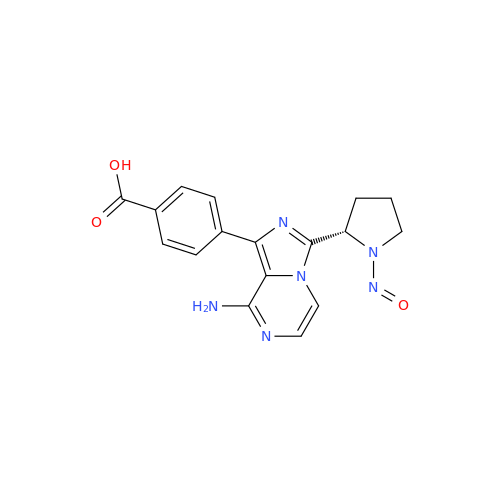
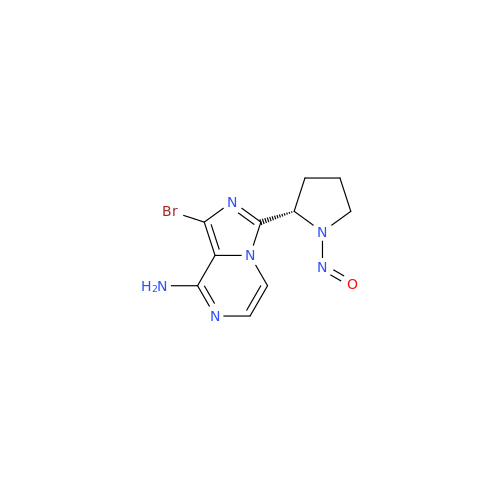
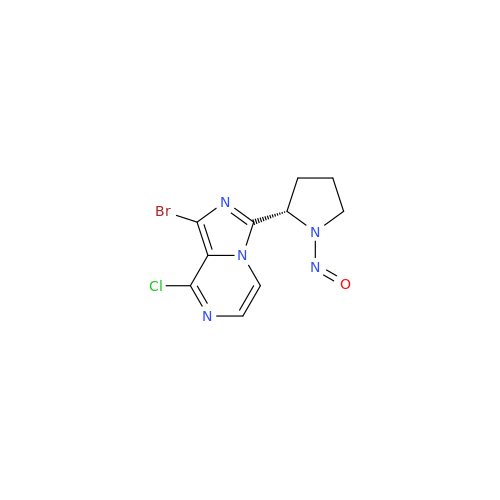
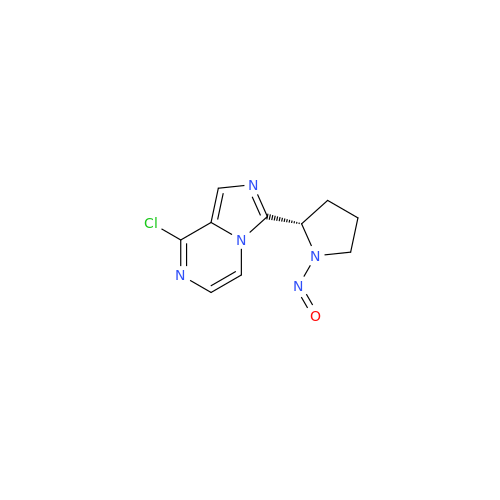
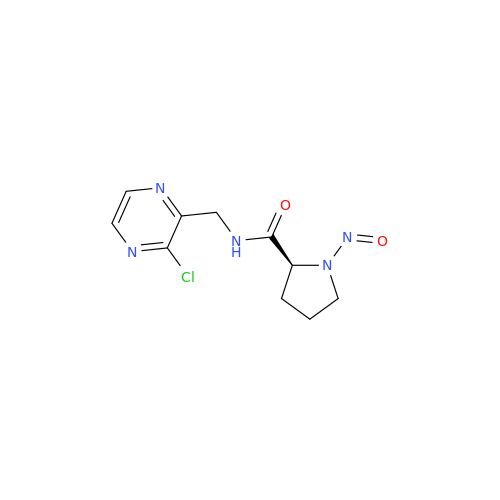
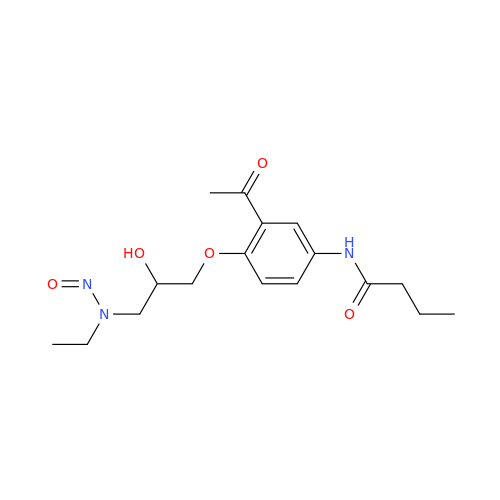
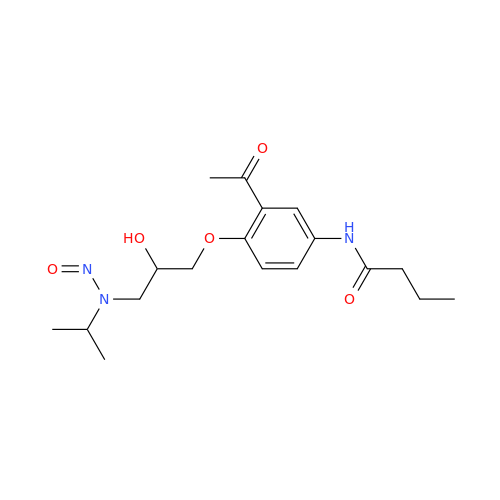
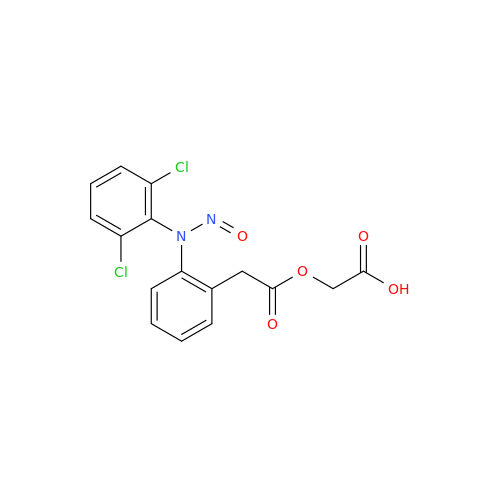
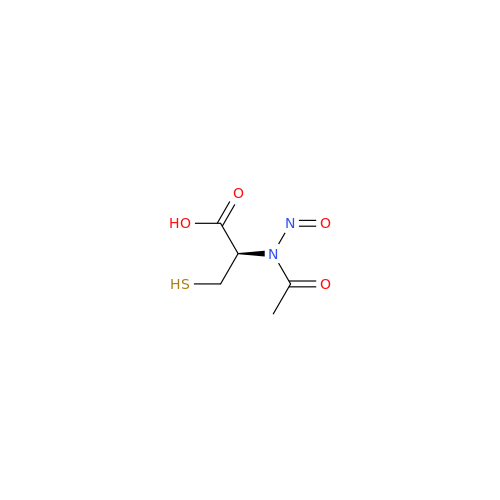
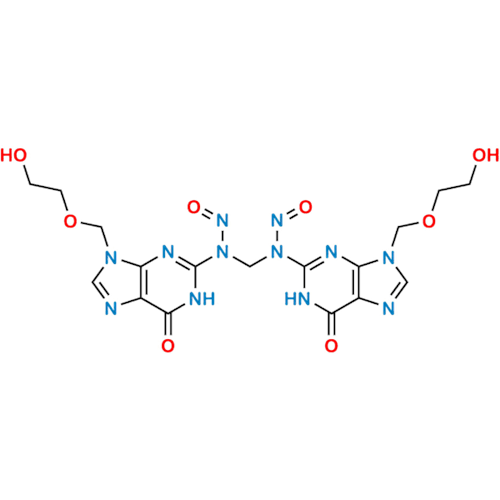
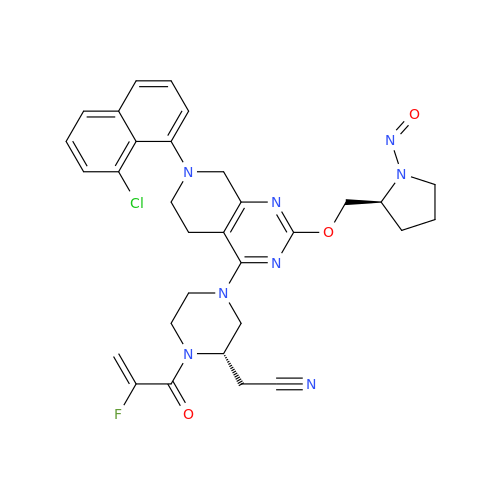
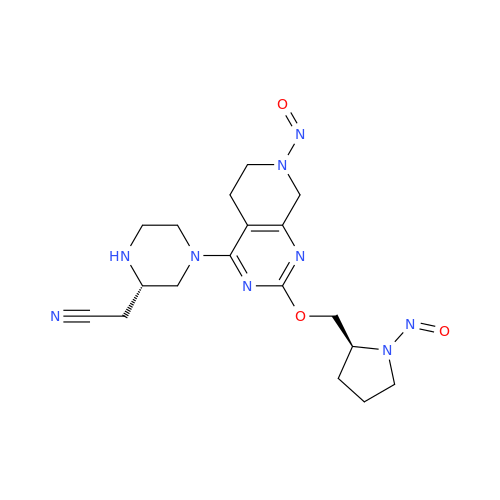
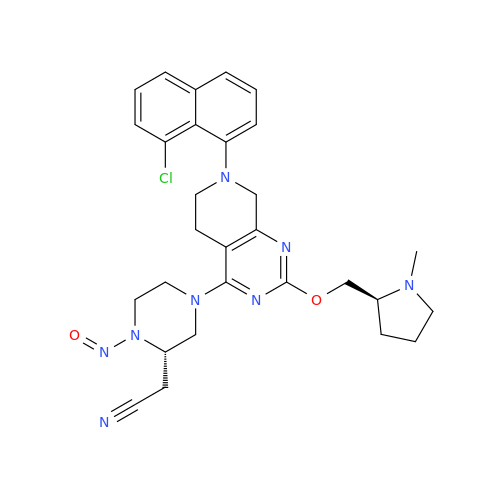
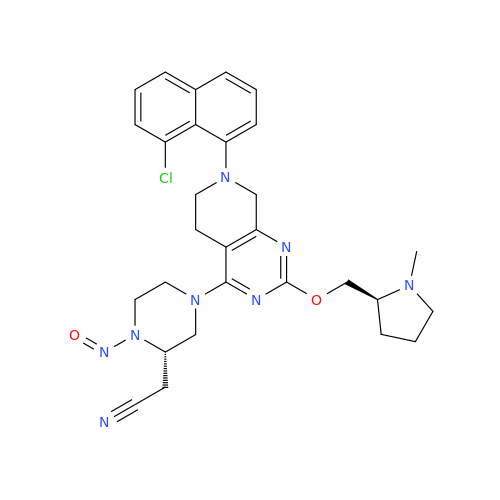
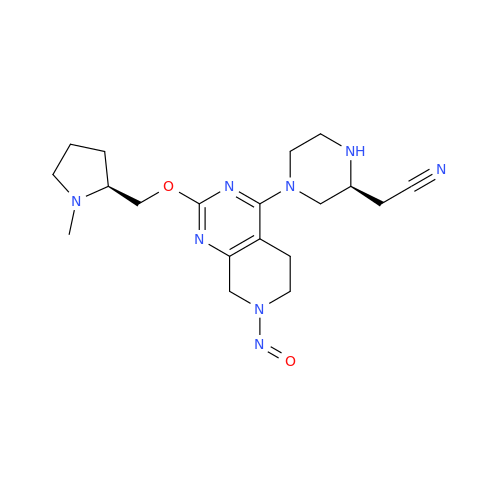
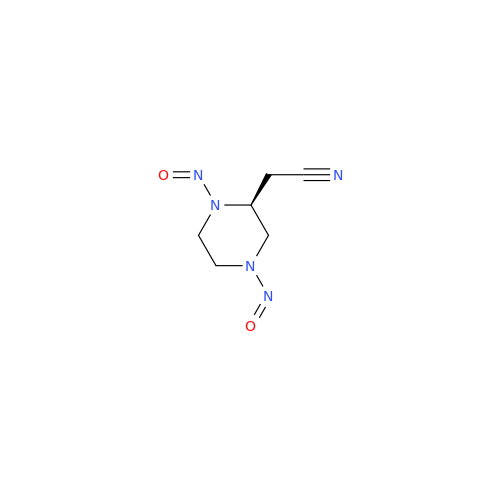
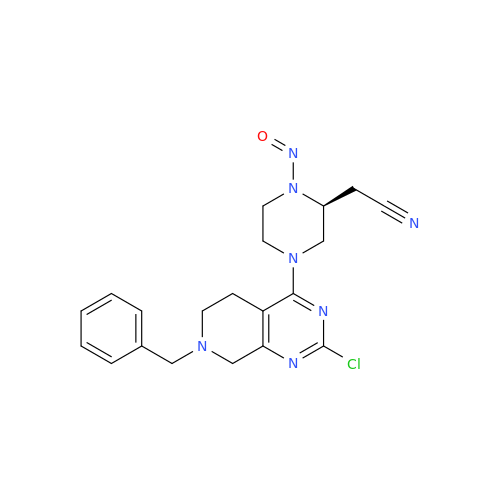
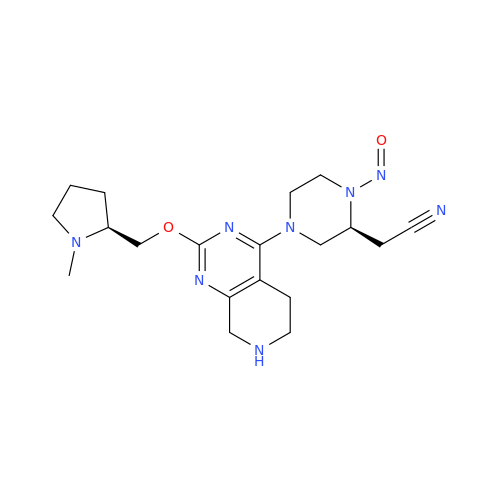
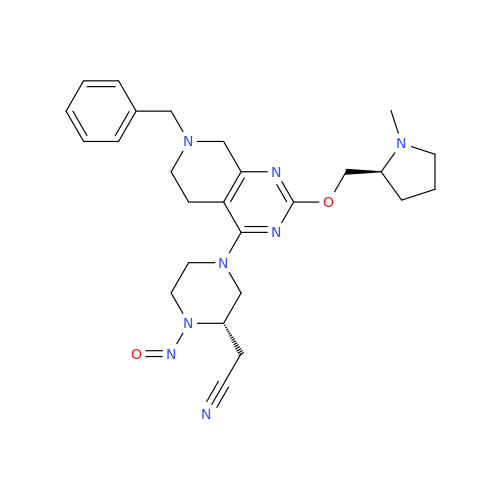
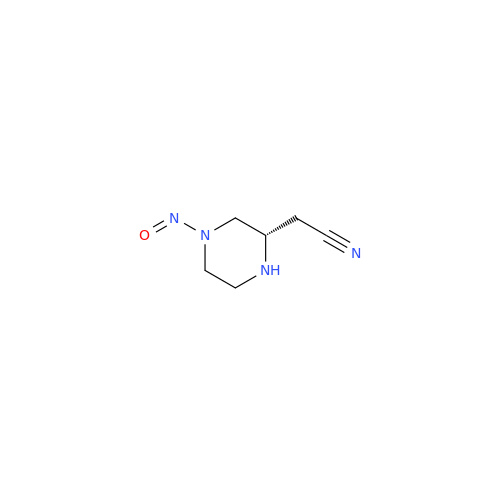
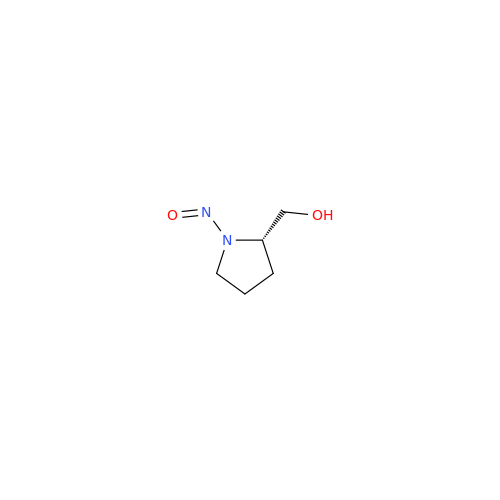
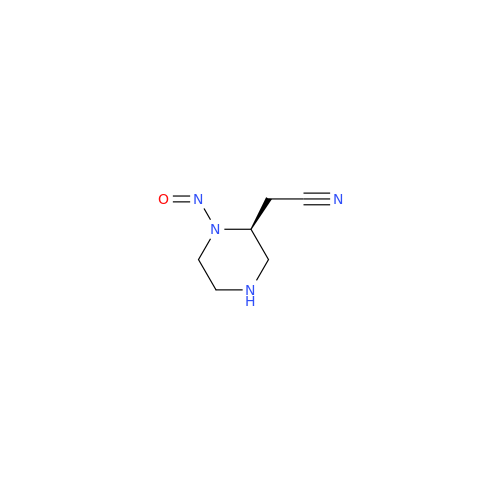
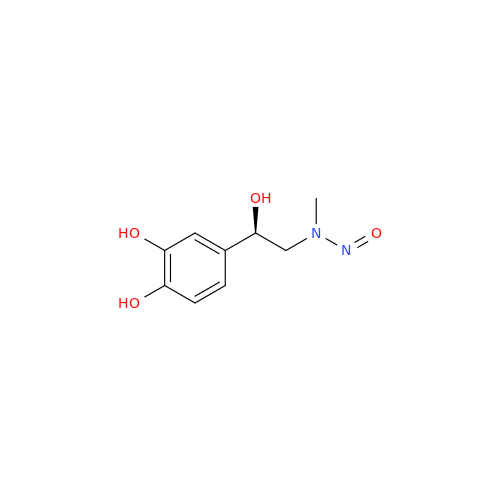
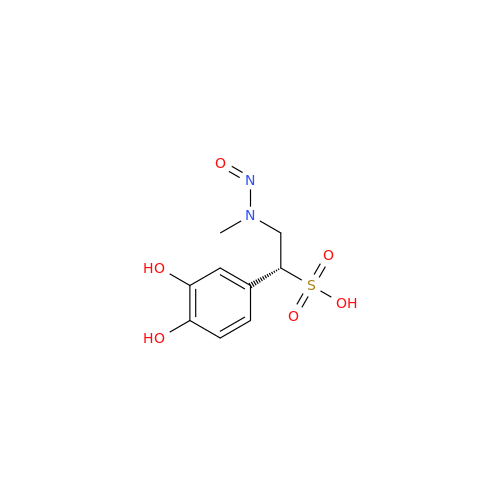
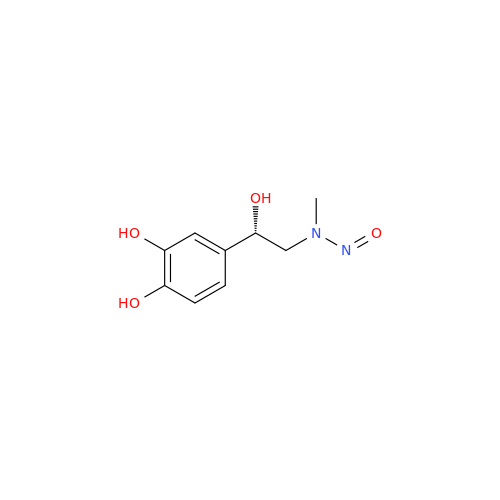
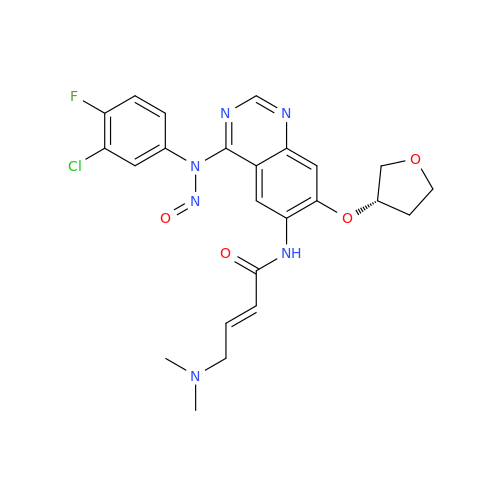
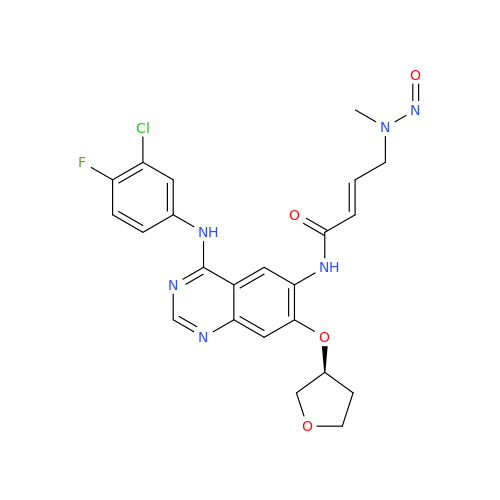
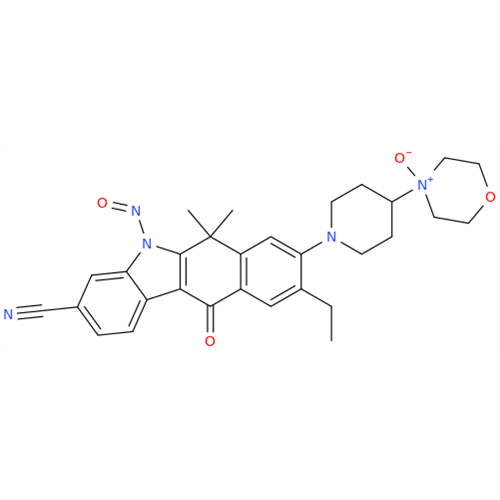
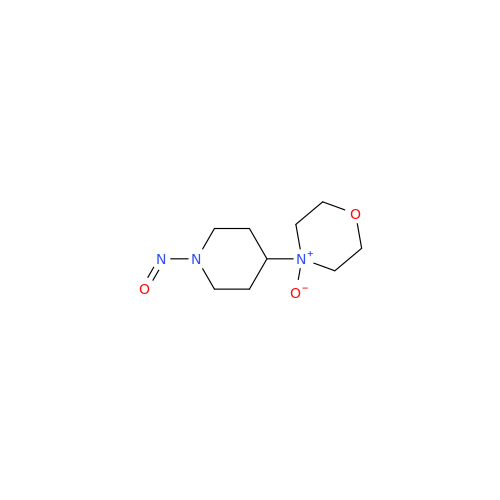
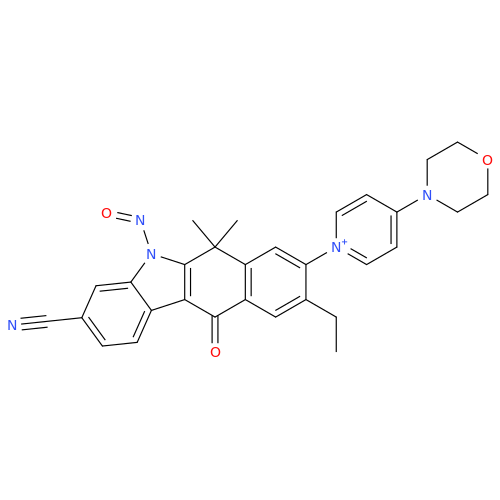
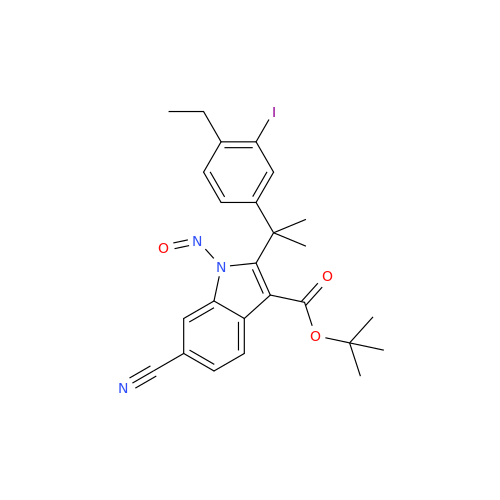
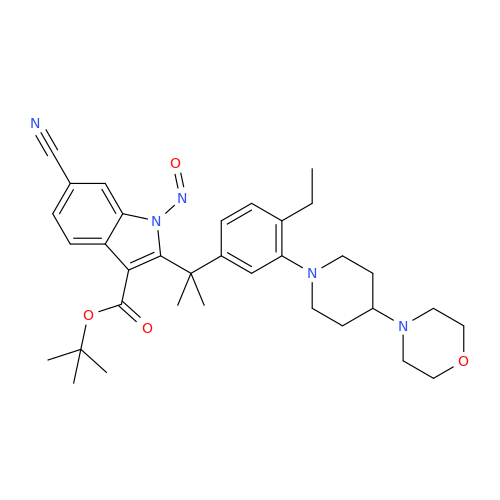
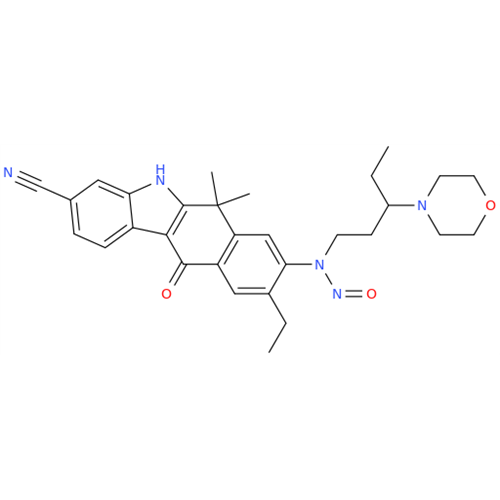
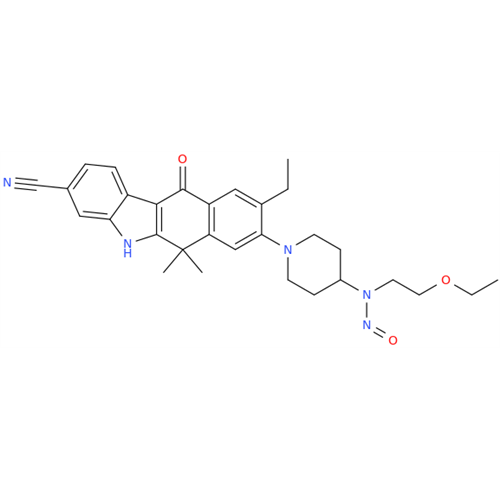
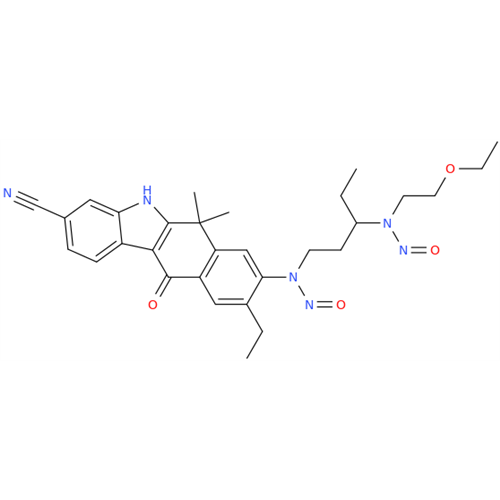
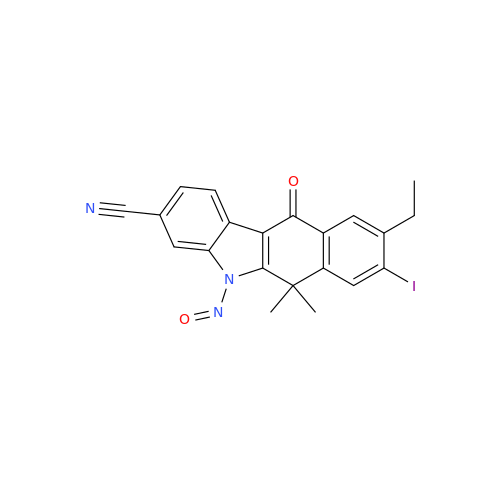
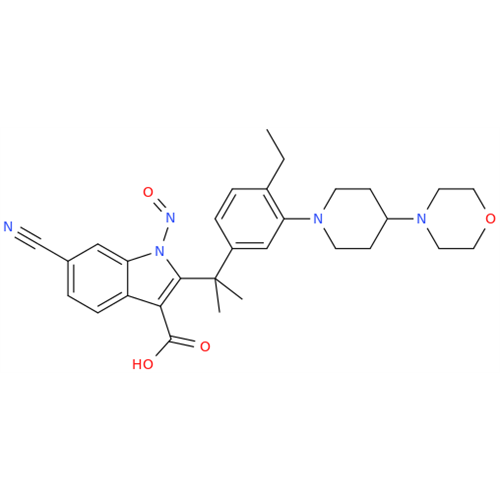
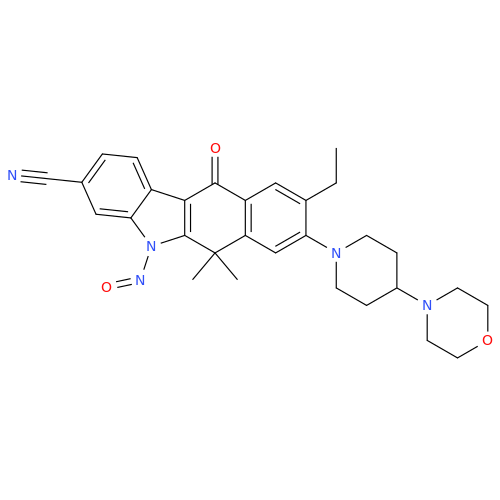
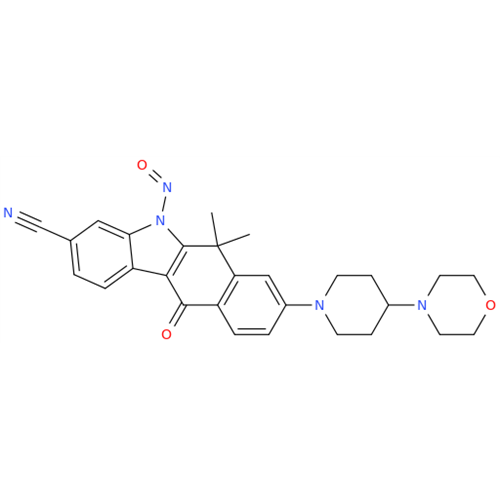
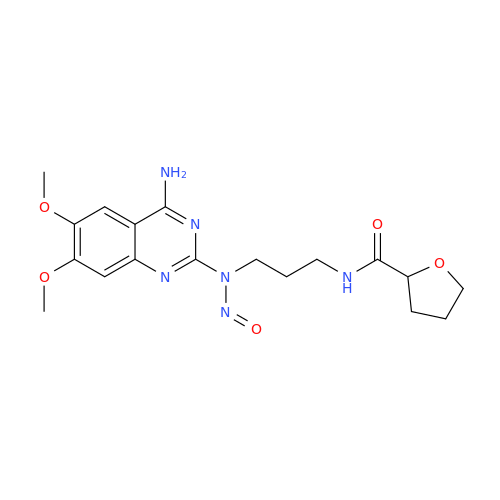
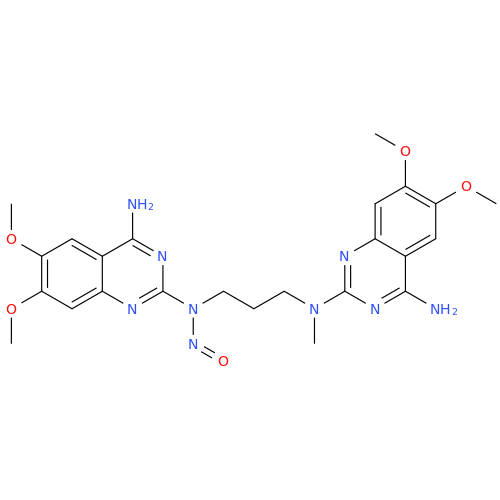
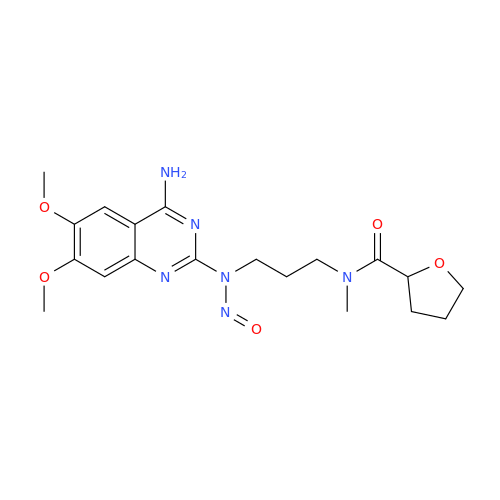
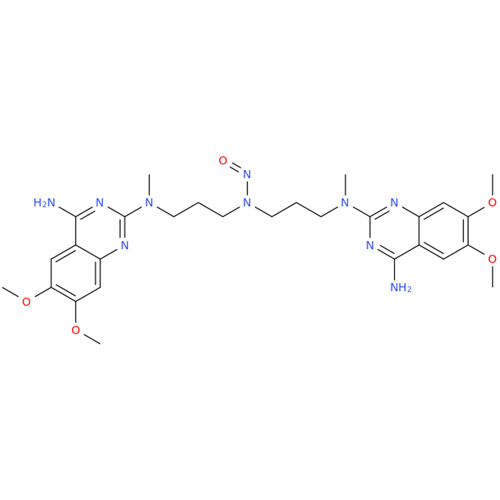
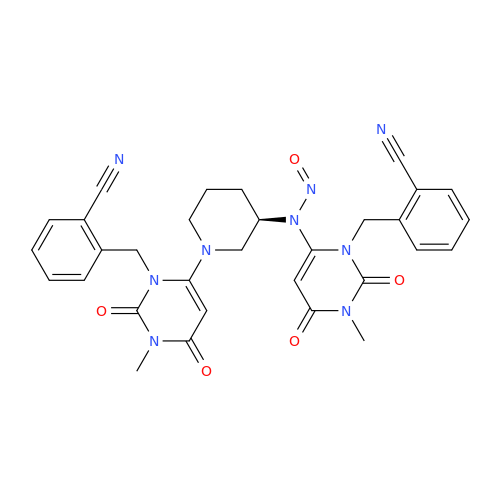
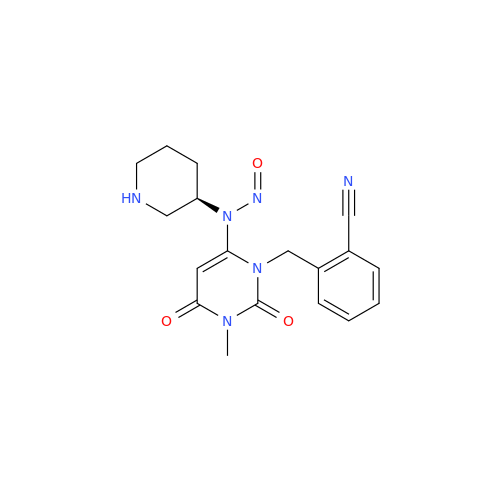
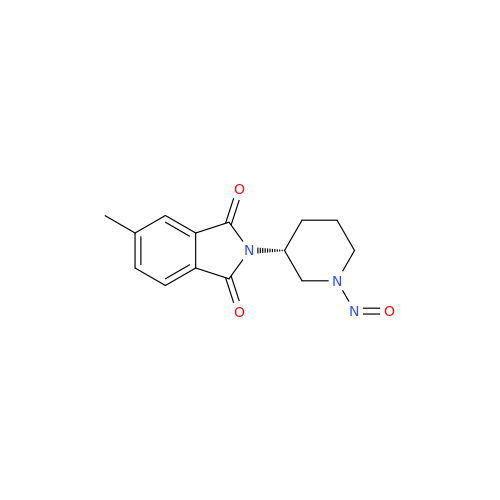
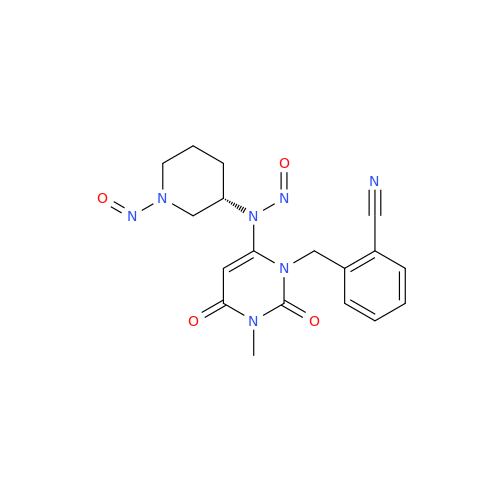
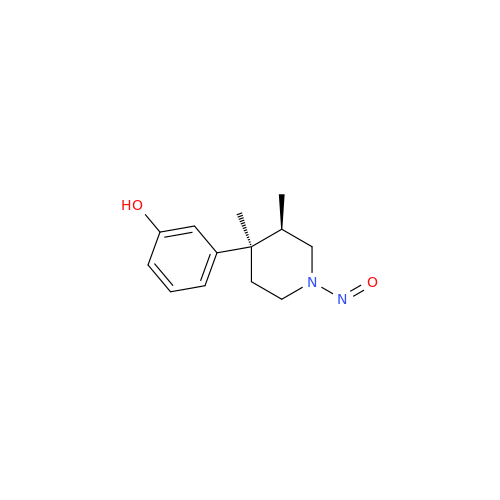
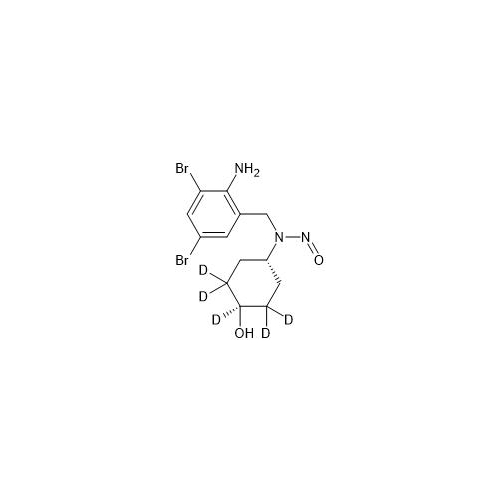
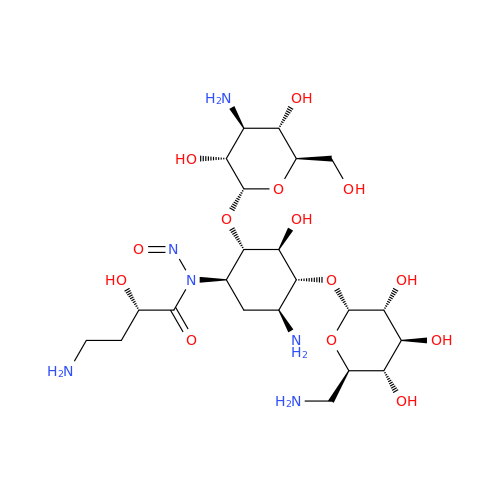
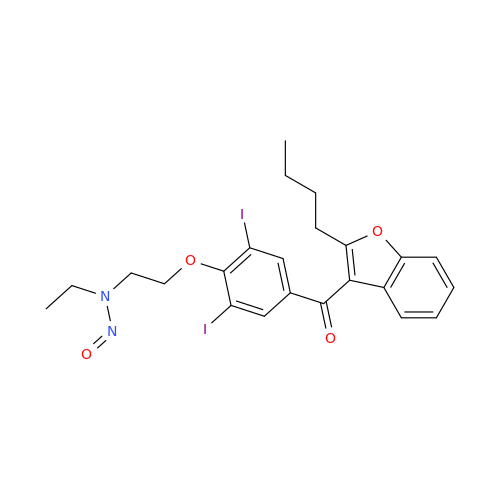
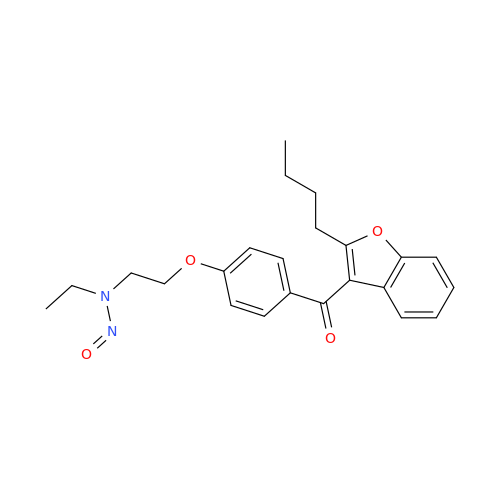
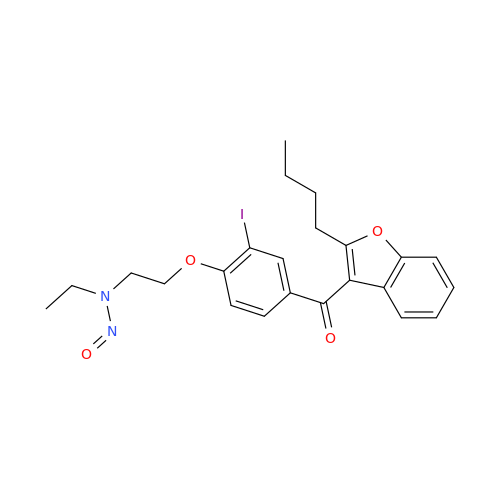
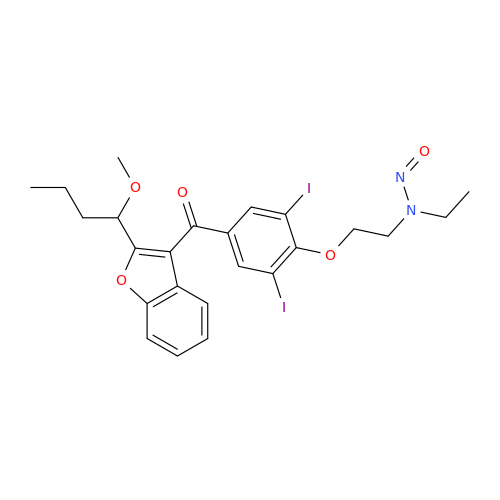
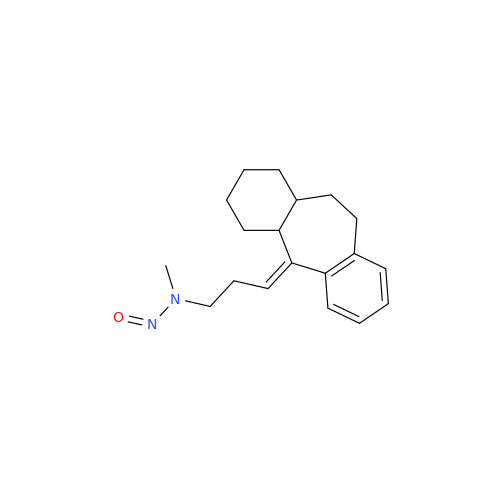
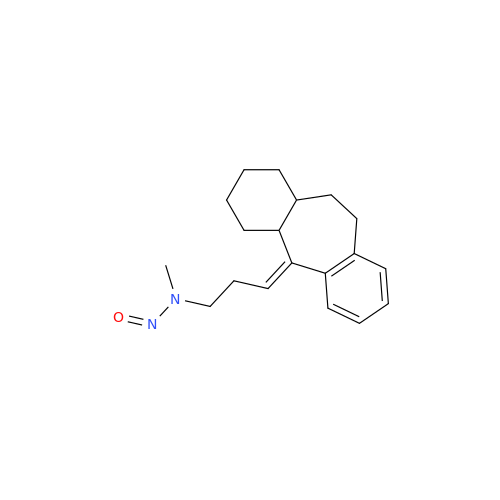
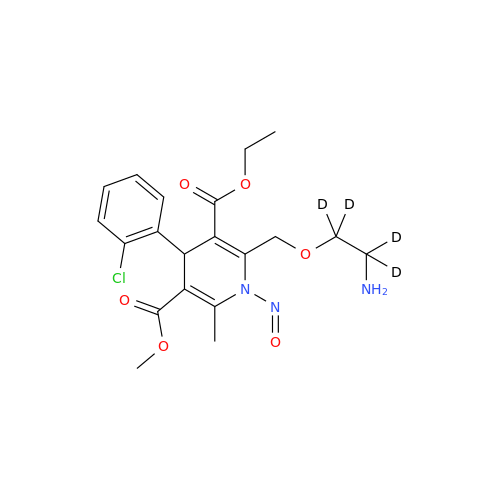
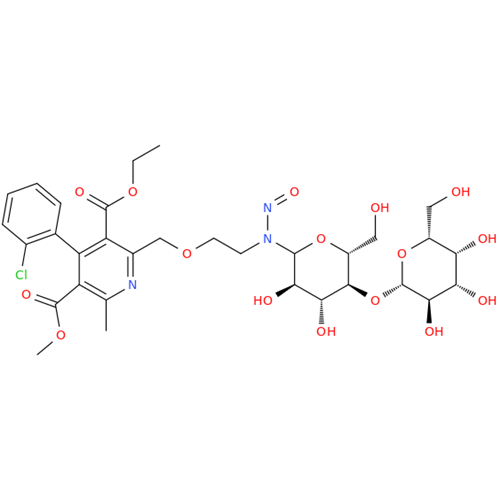
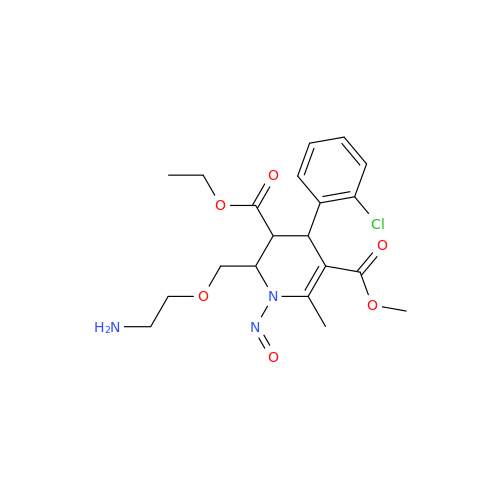
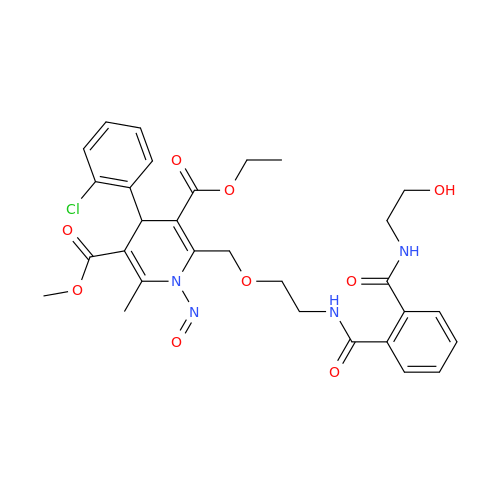
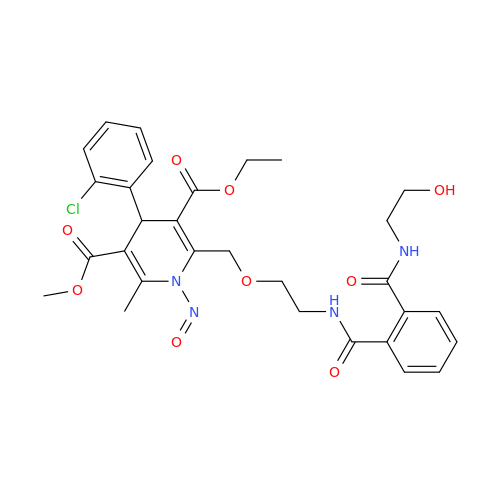
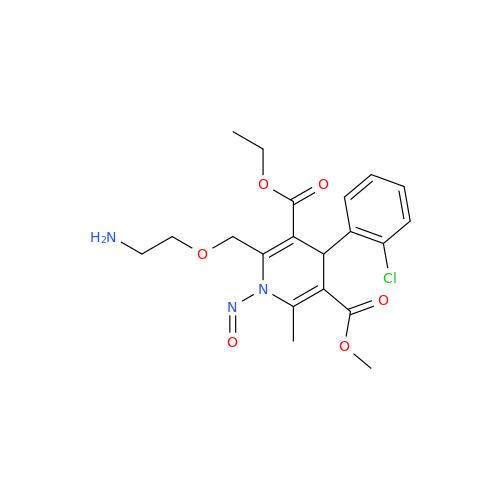
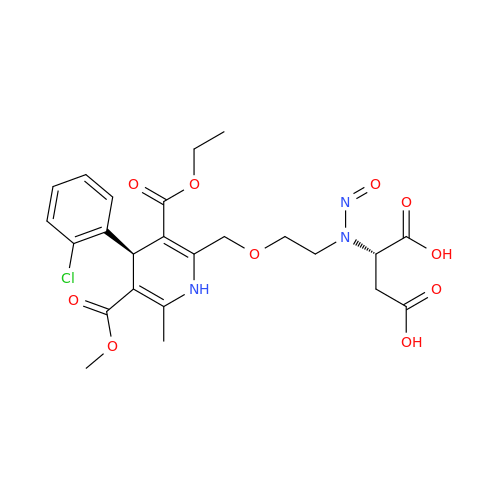
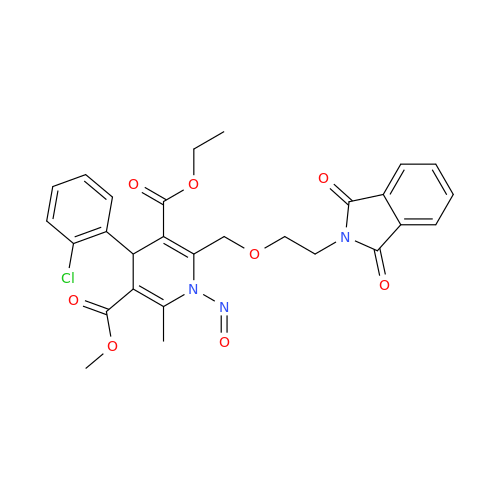
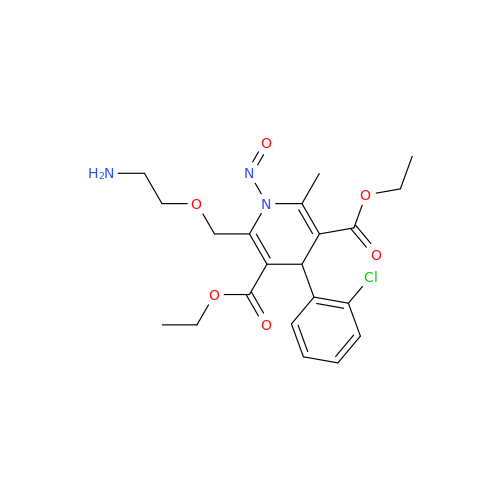
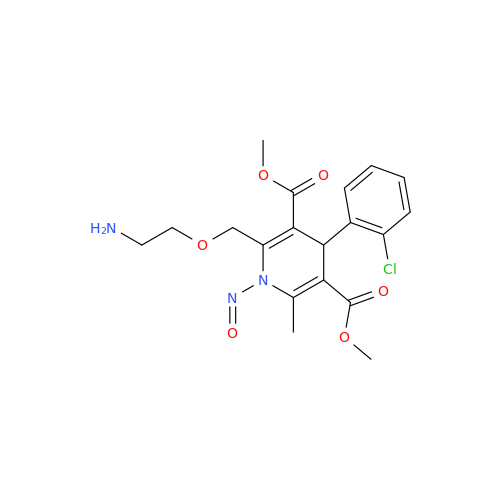
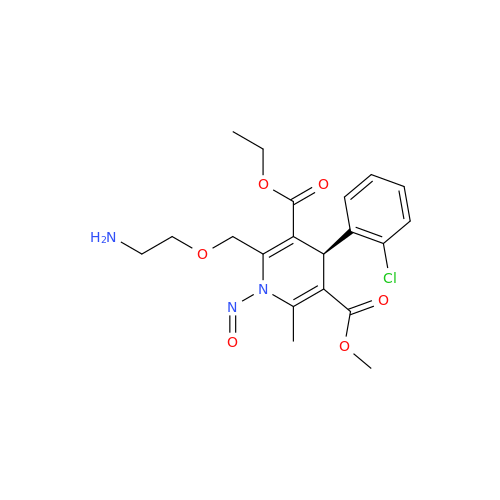
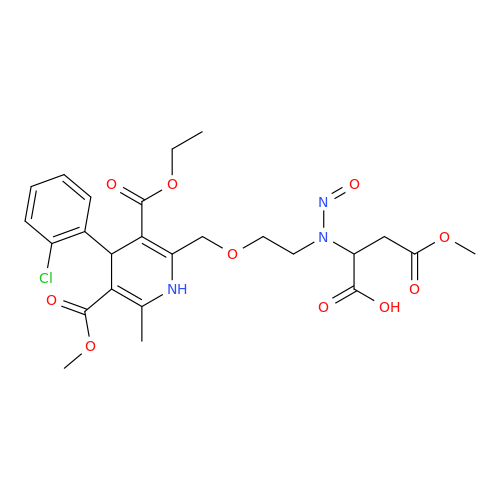
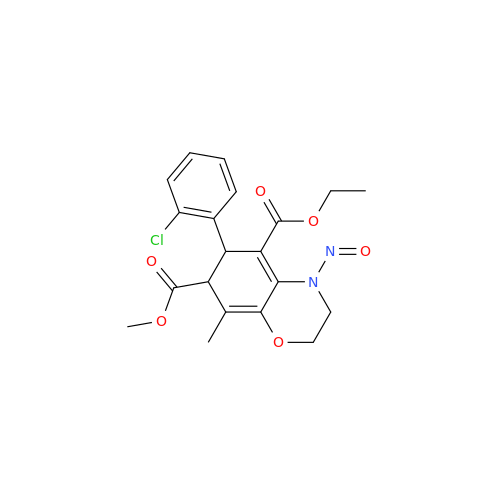
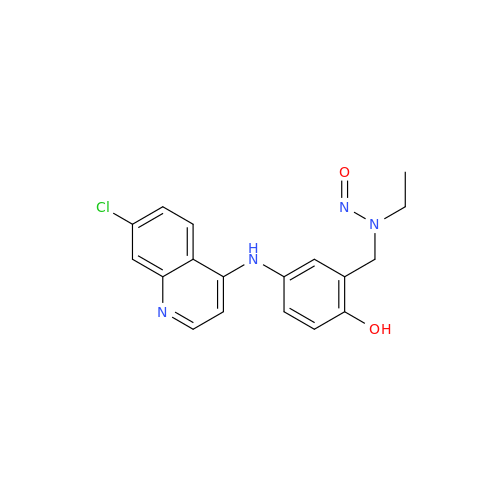
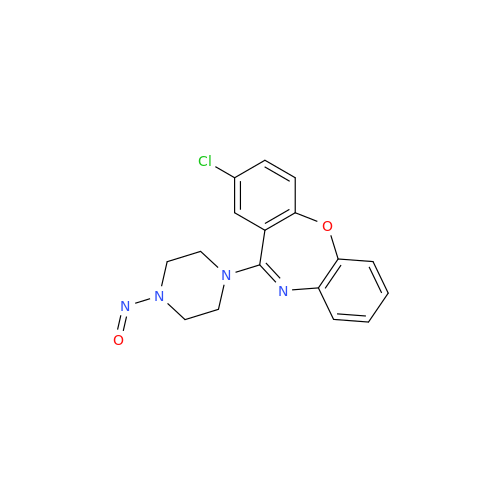
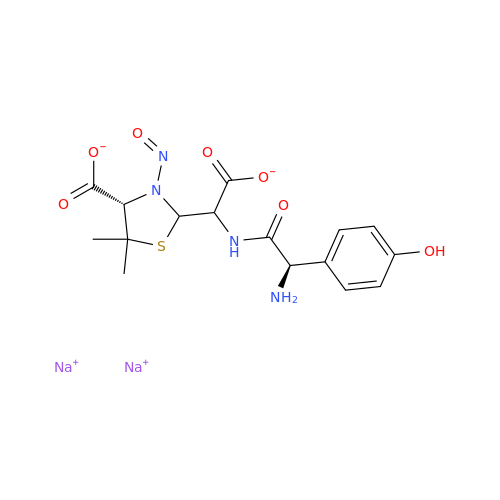
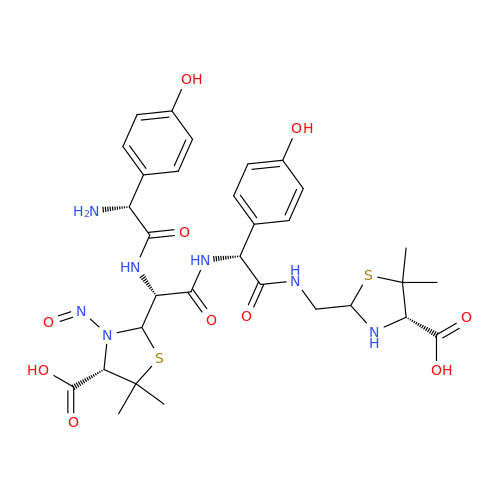
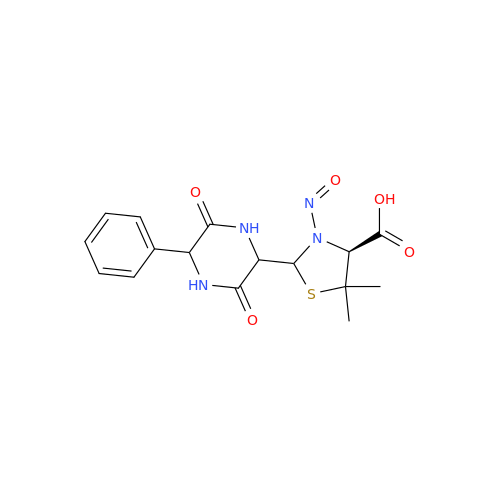
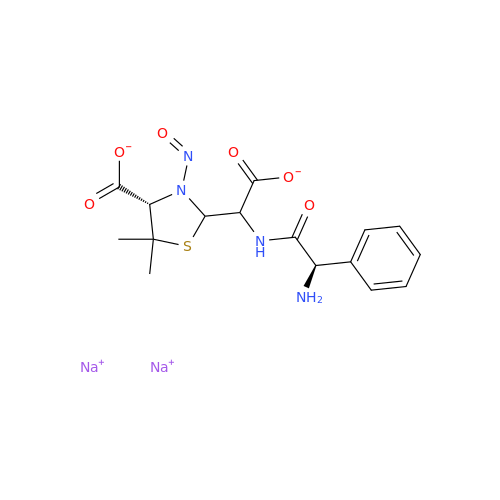
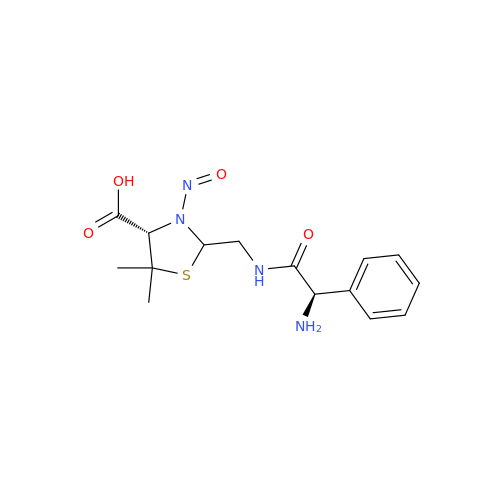
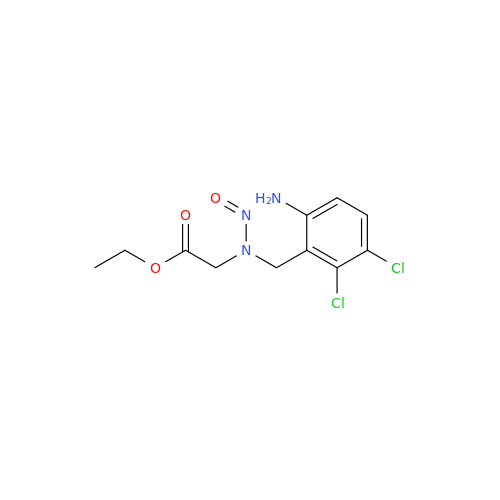
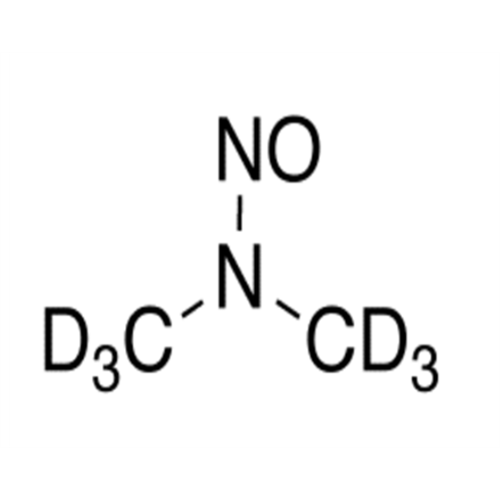
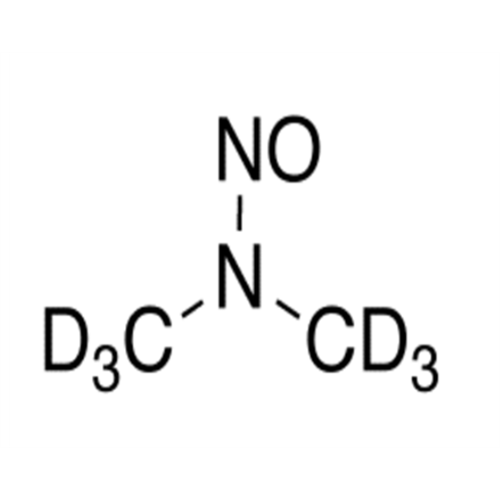
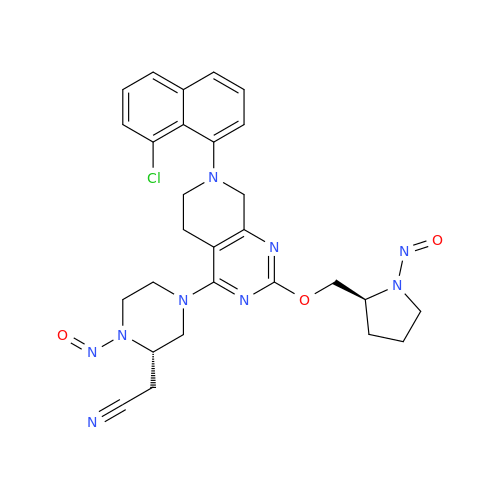
Adagrasib N-Nitroso Impurity 6
Add to Cart
Bulk Enquiry
|
Chemical Name: Adagrasib N-Nitroso Impurity 6
Synonym: 2-((S)-4-(7-(8-chloronaphthalen-1-yl)-2-(((S)-1-nitrosopyrrolidin-2-yl)methoxy)-5,6,7,8-tetrahydropyrido[3,4-d]pyrimidin-4-yl)-1-nitrosopiperazin-2-yl)acetonitrile