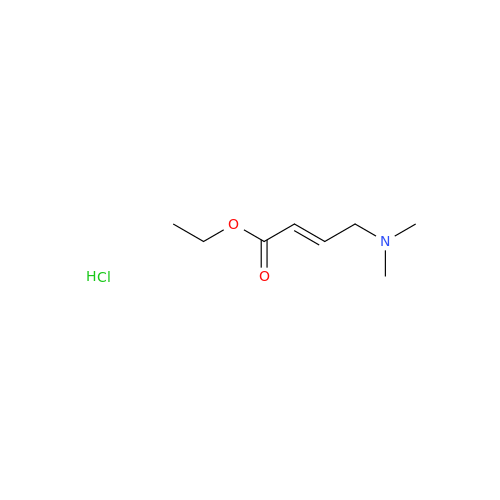
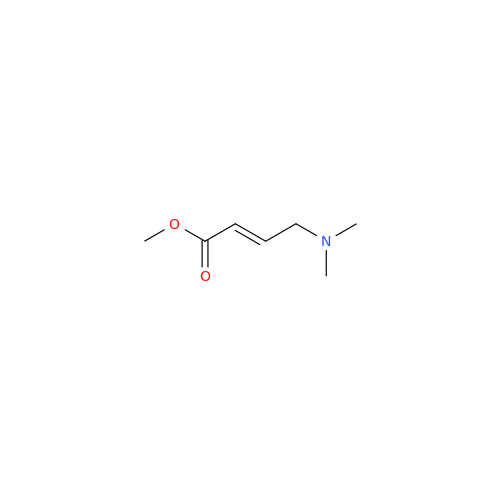
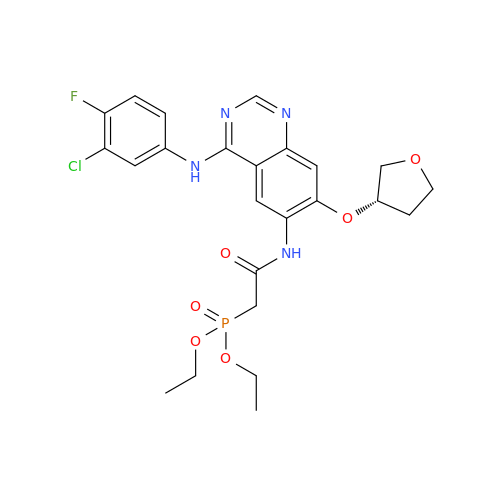
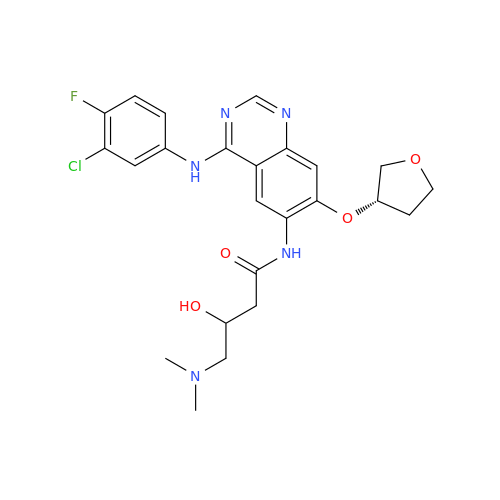
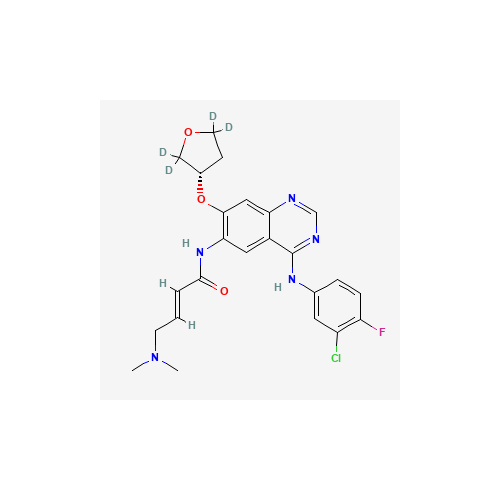
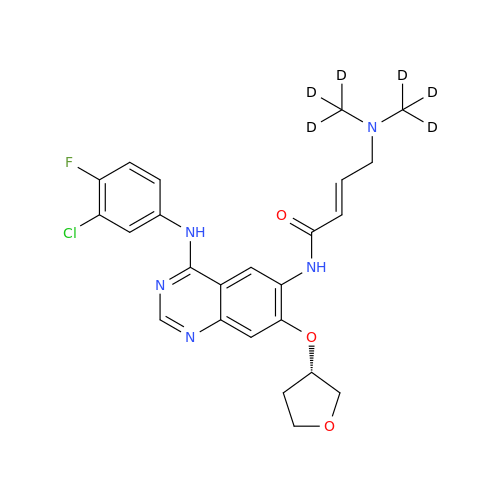
Product Information
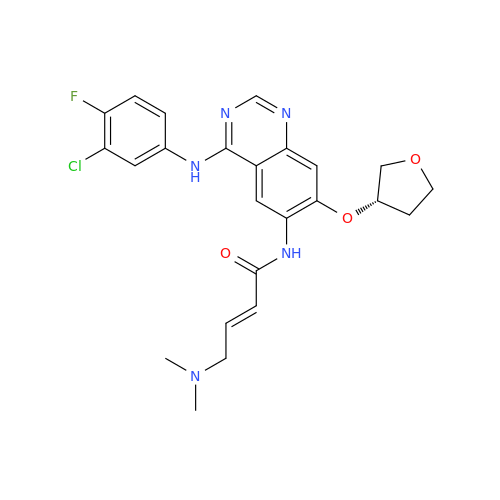
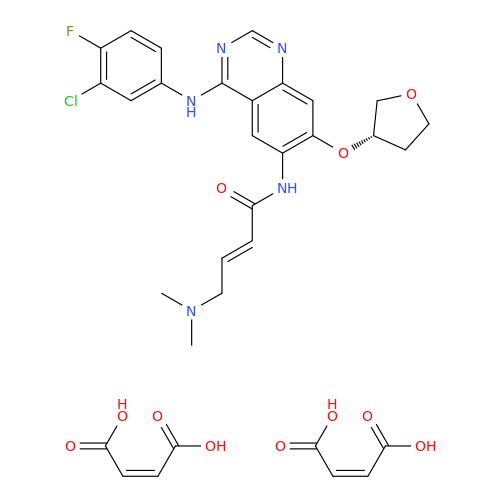
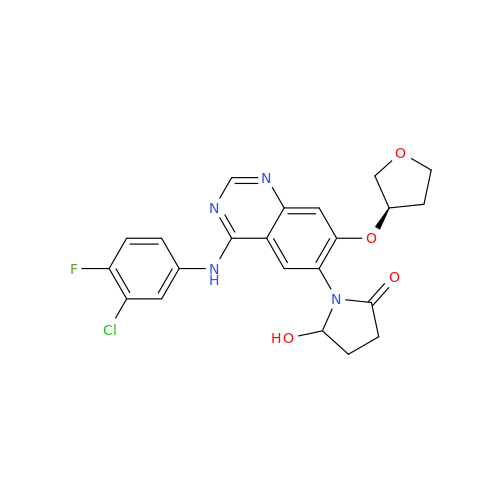
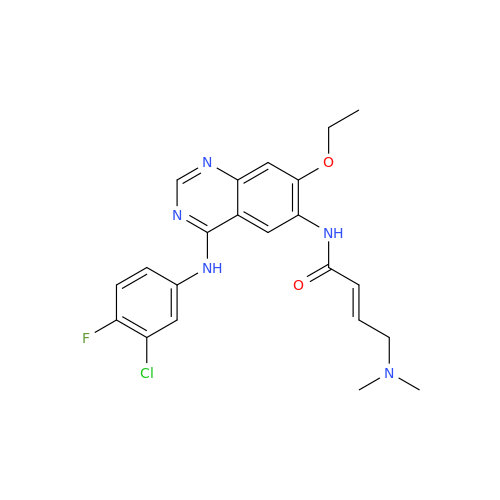
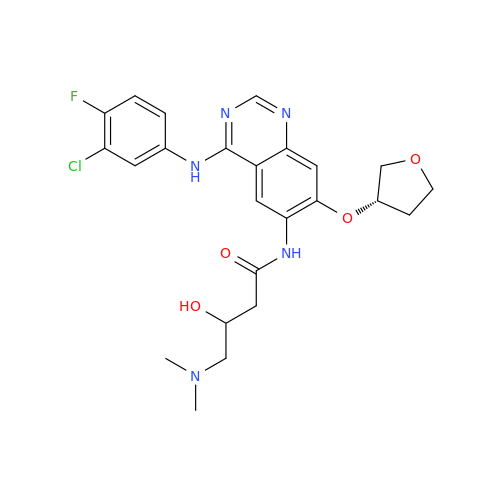
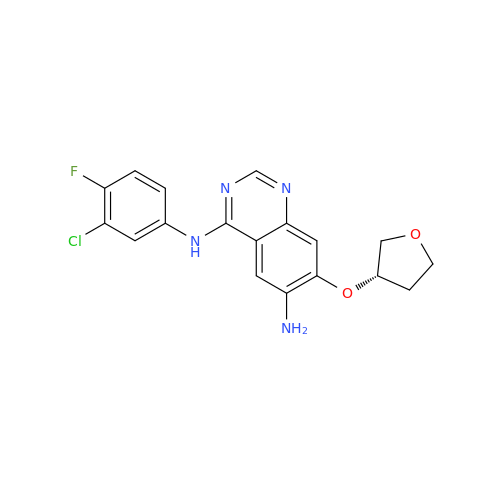
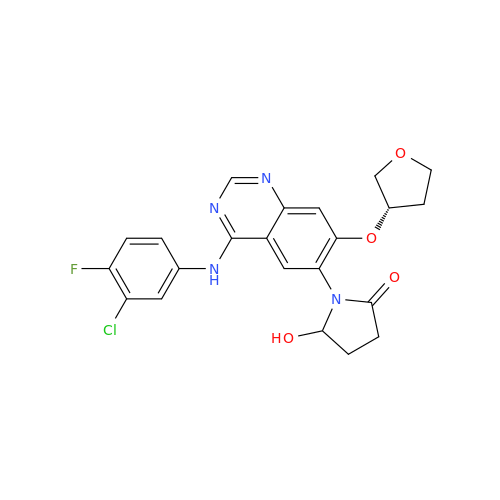
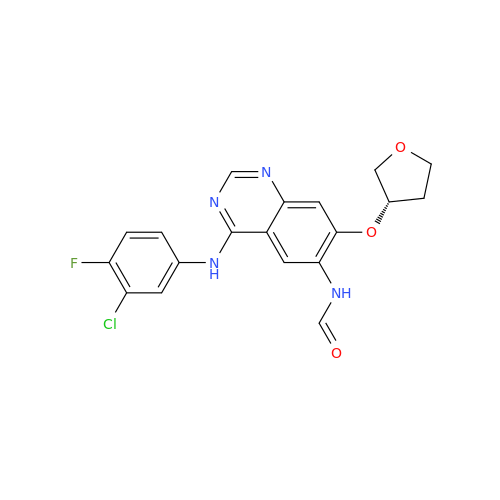
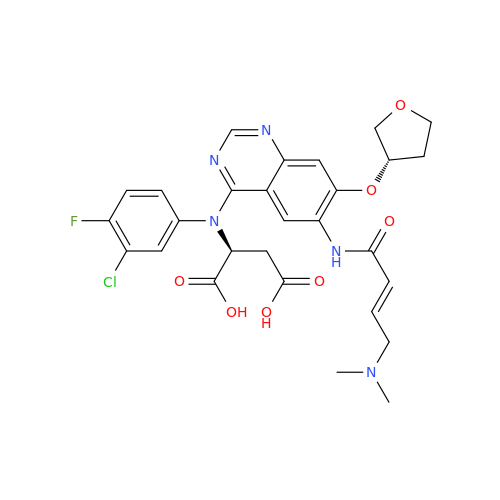
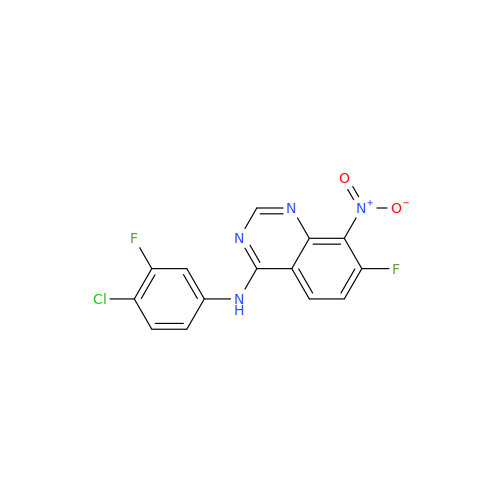
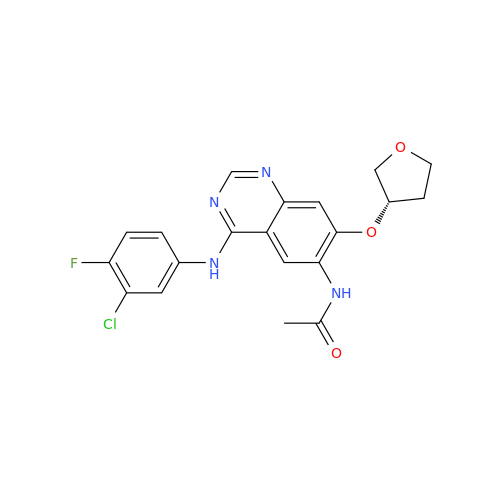
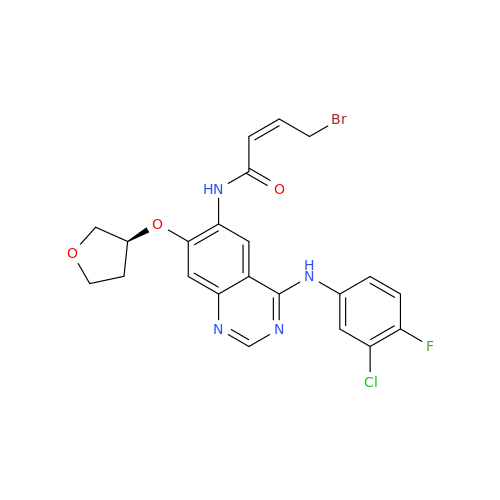
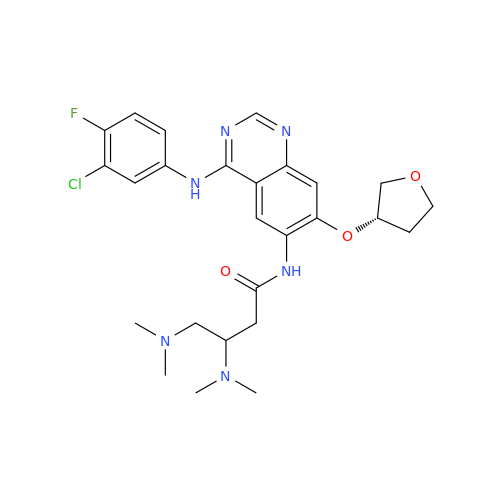
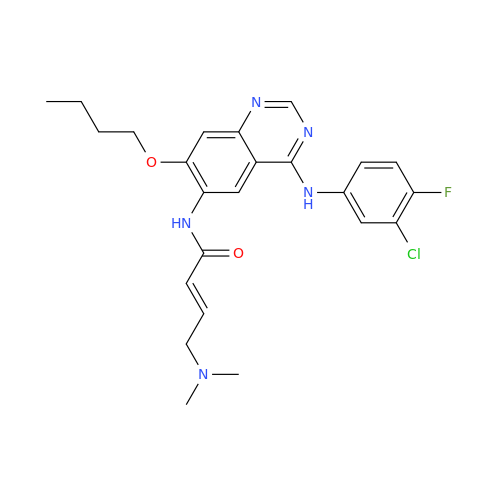
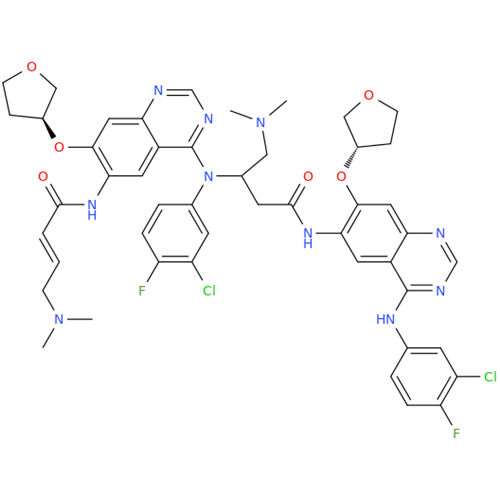
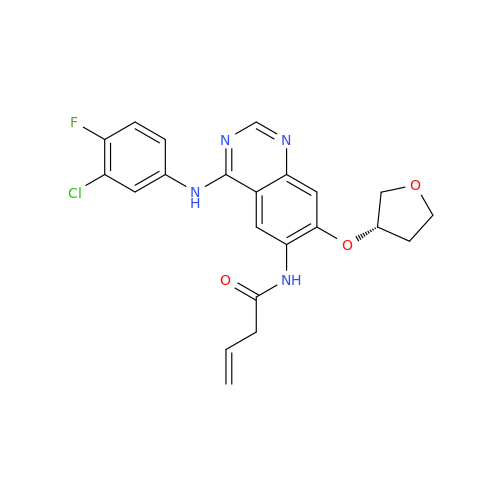
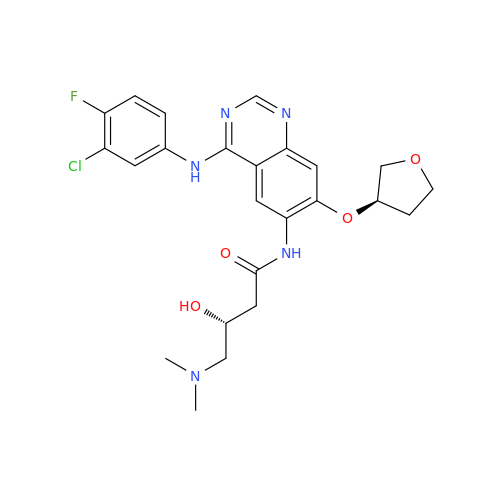
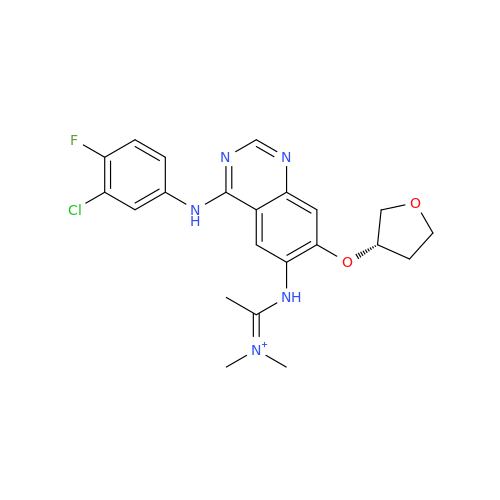
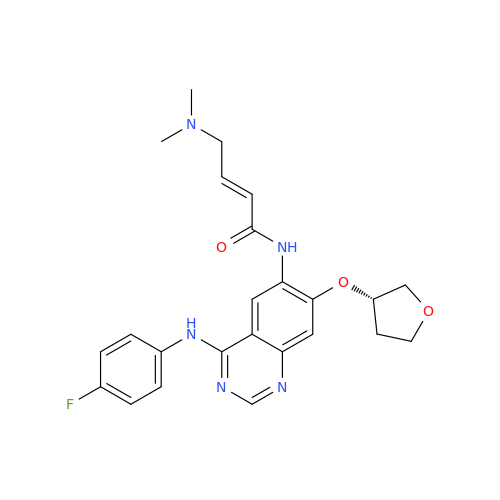
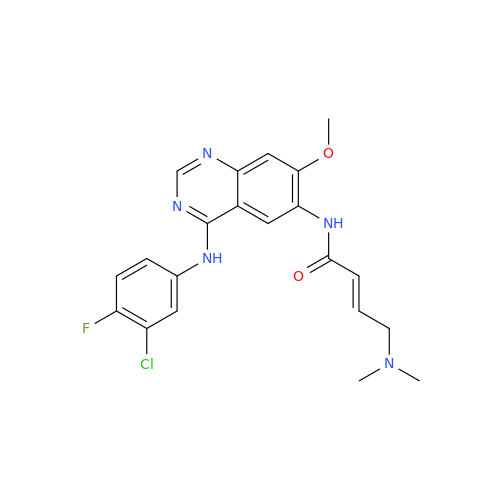
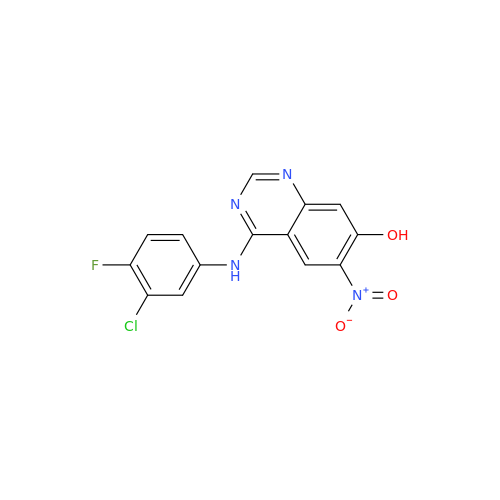
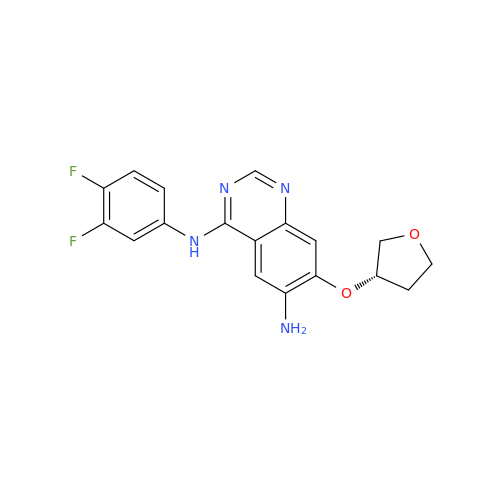
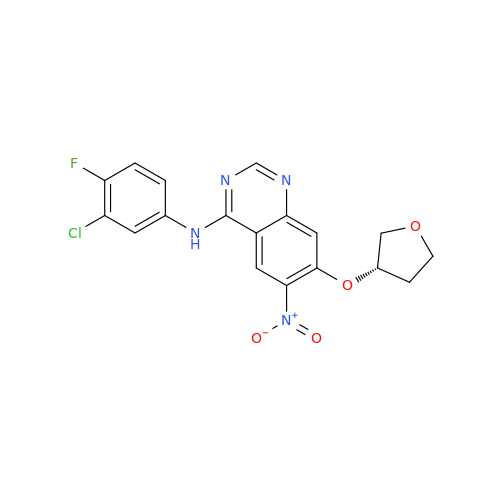
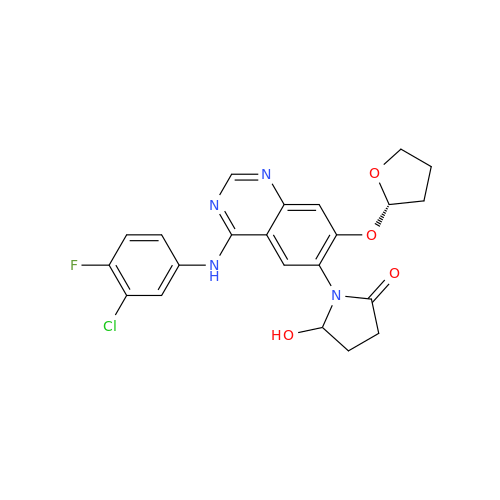
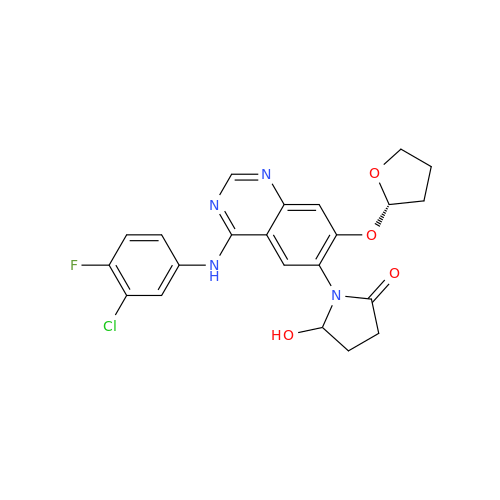
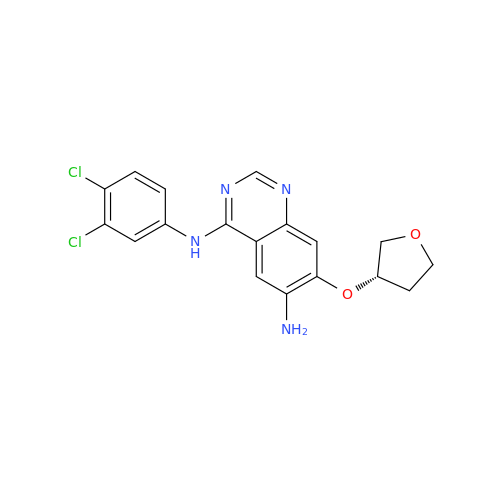
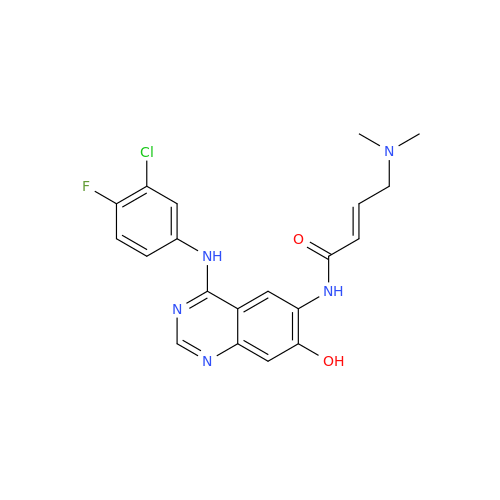
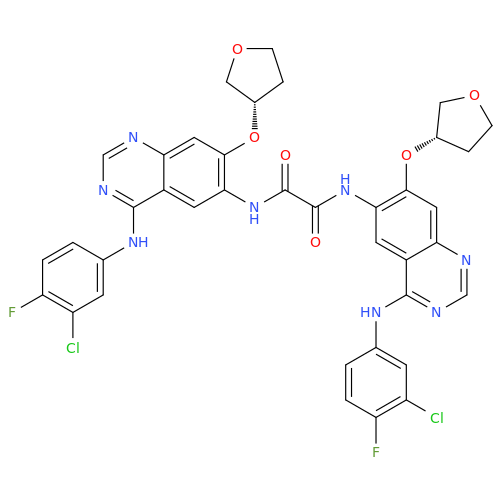
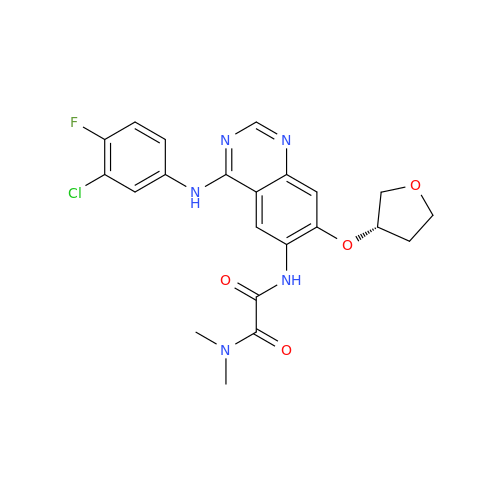
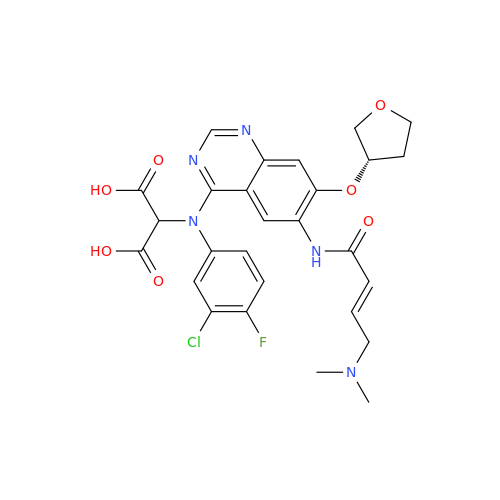
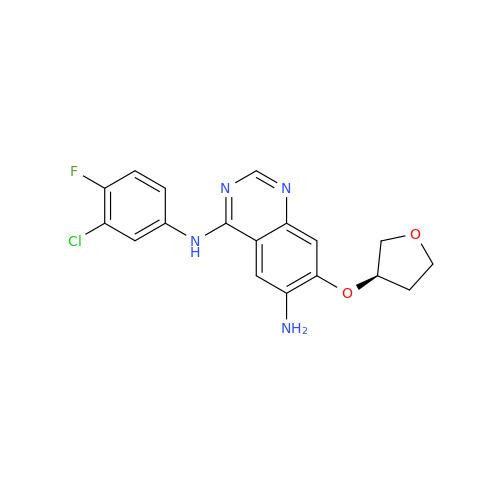
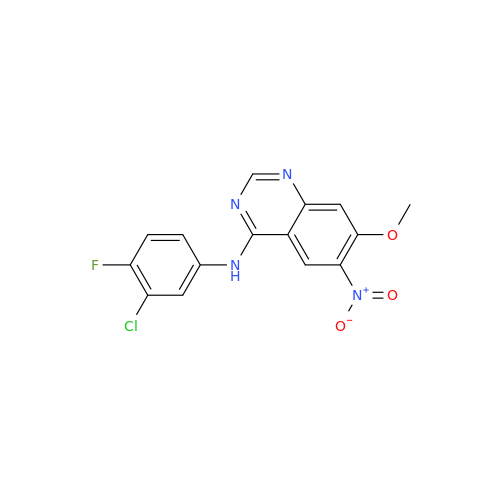
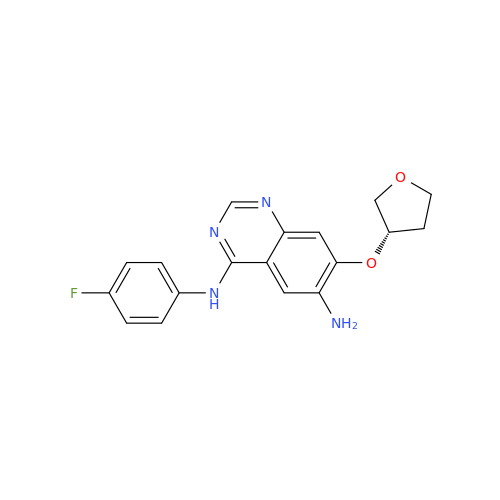
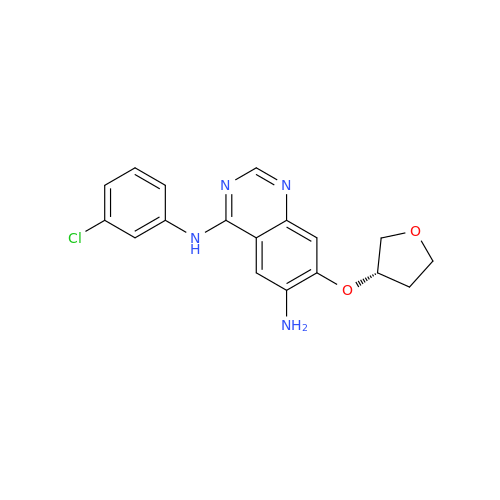
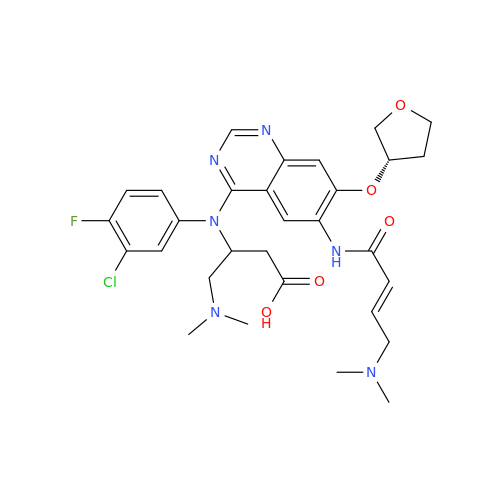
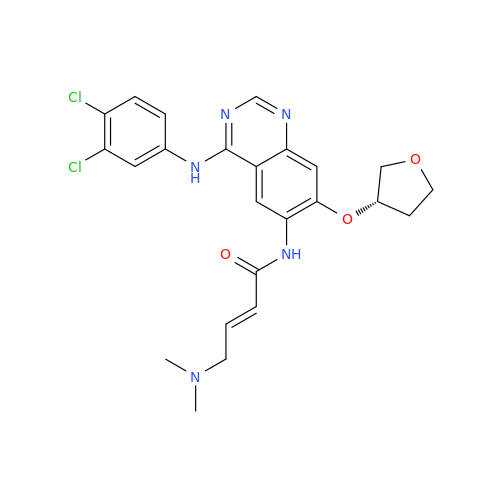
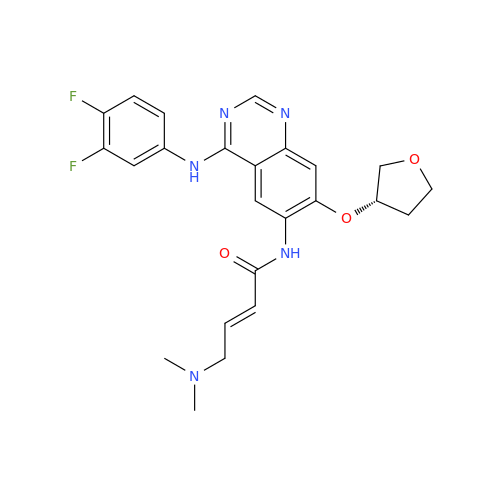
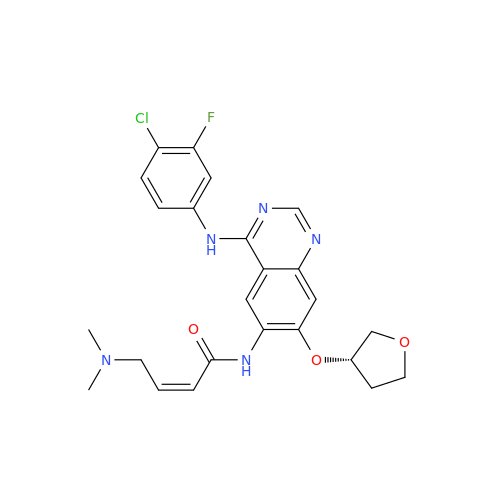
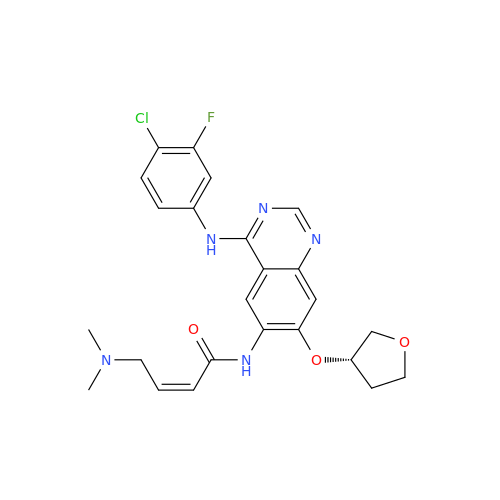
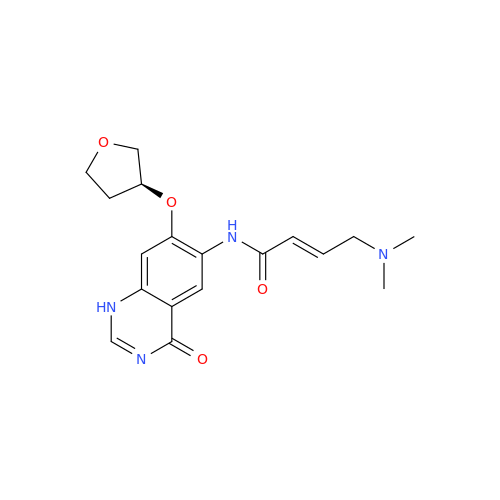
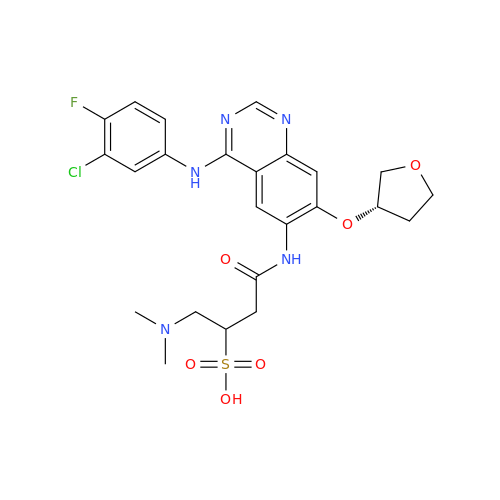
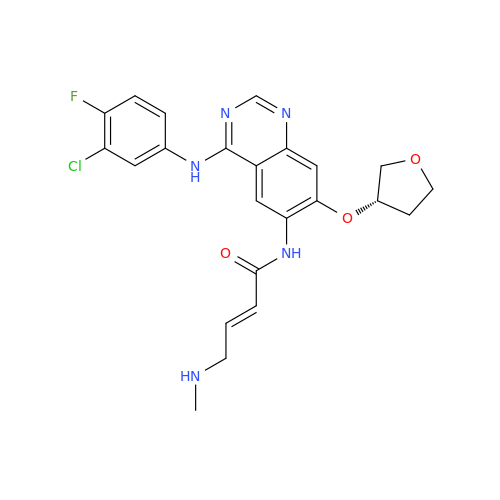
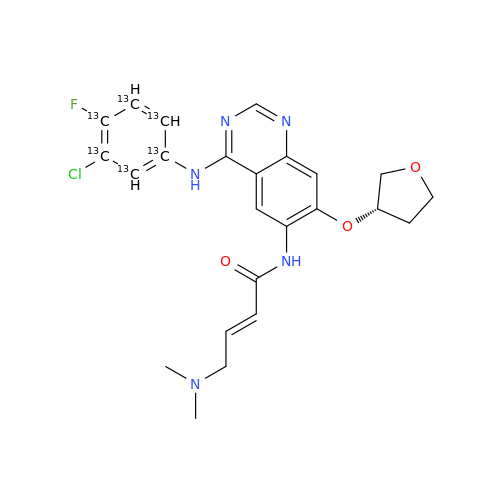
Afatinib Impurity 7
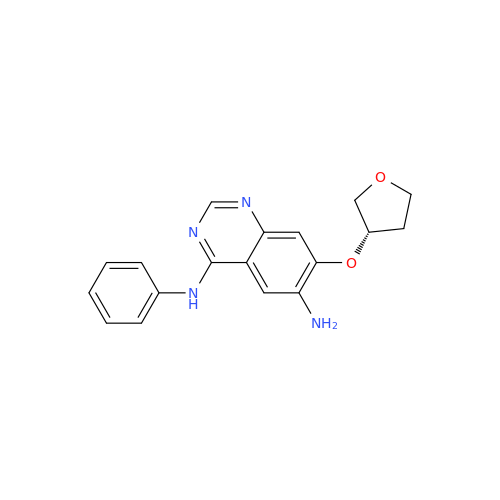
|
Chemical Name: Afatinib Impurity 7
Synonym: 4,6-Quinazolinediamine, N4-phenyl-7-[[(3S)-tetrahydro-3-furanyl]oxy]| Enter Batch Number | |||