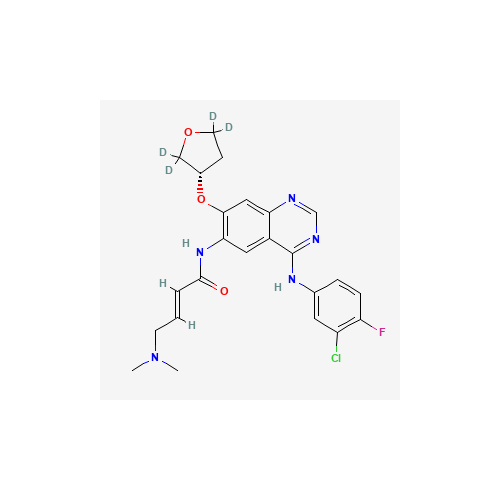
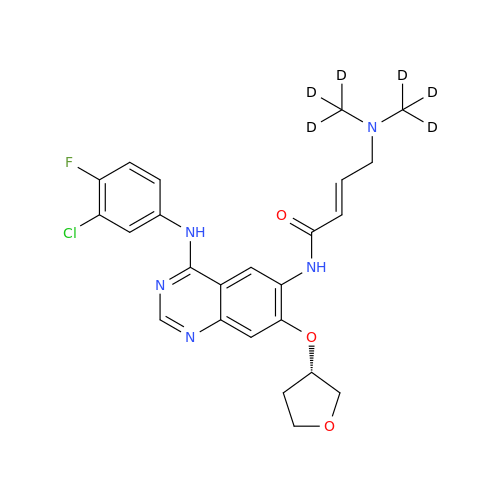
Product Information
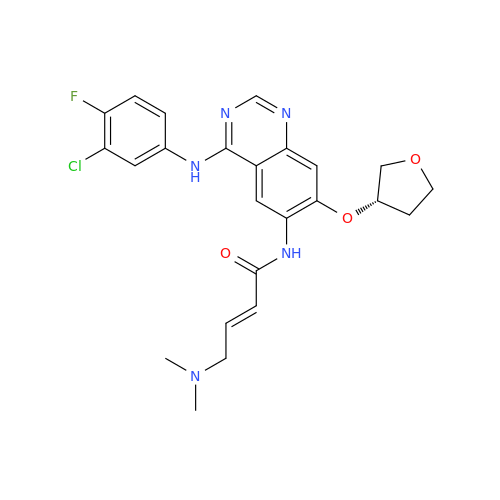
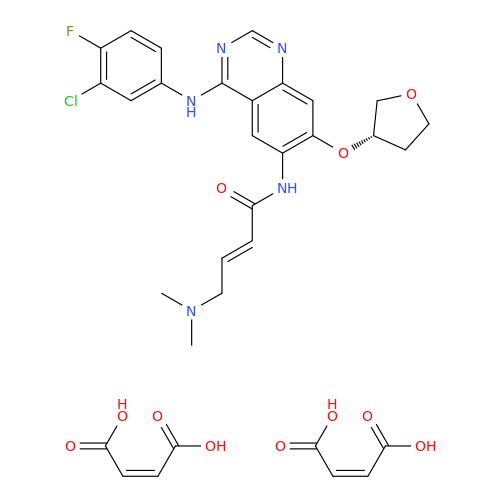
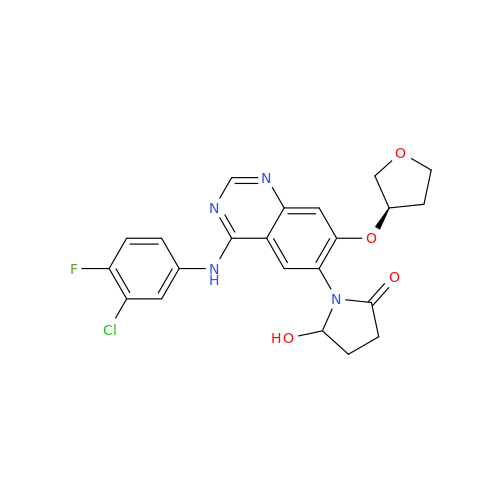
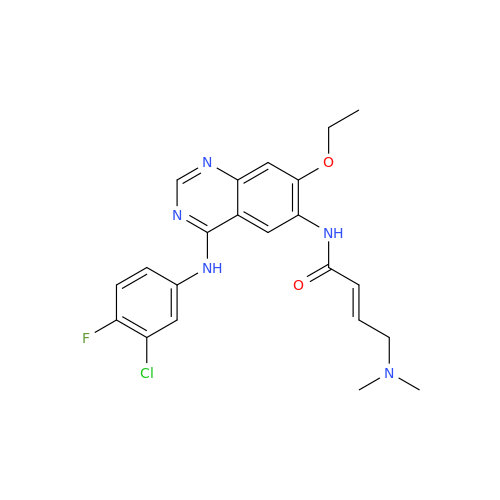
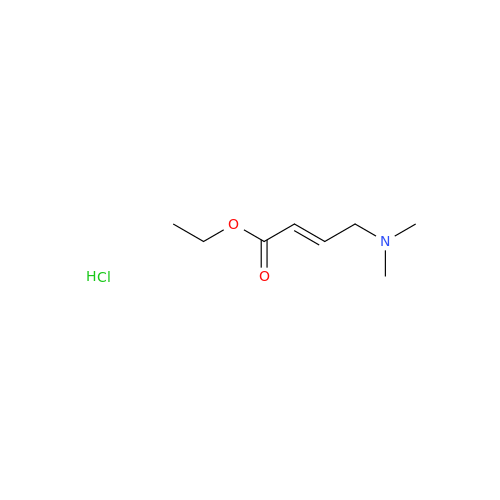
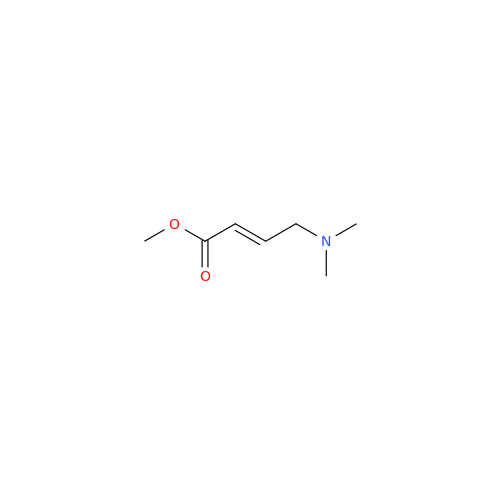
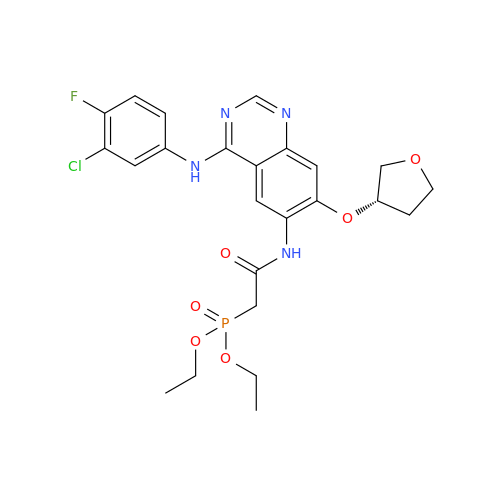
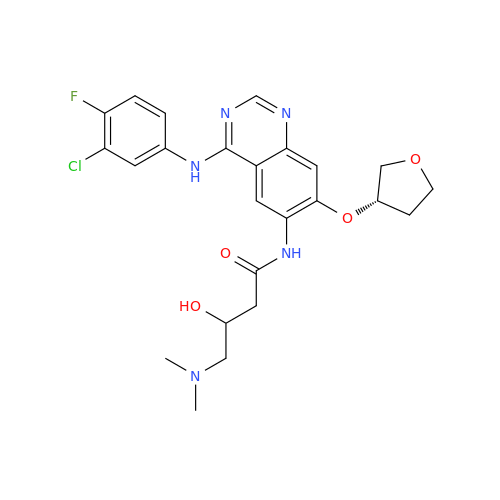
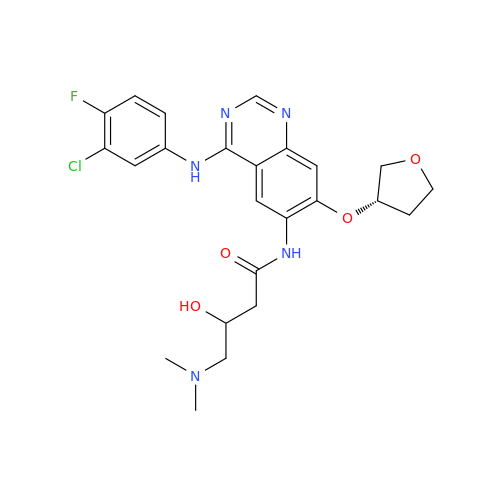
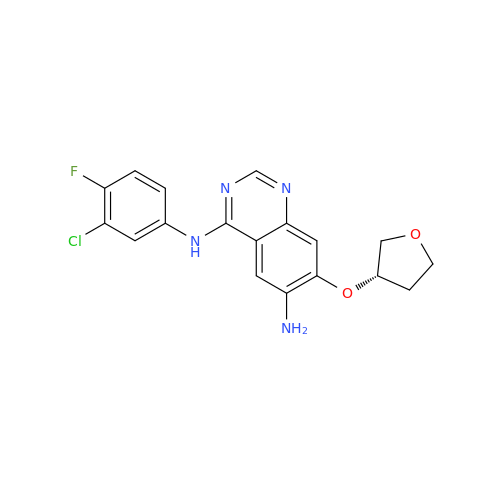
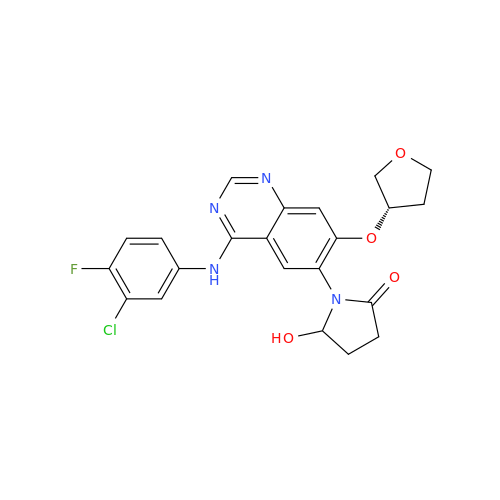
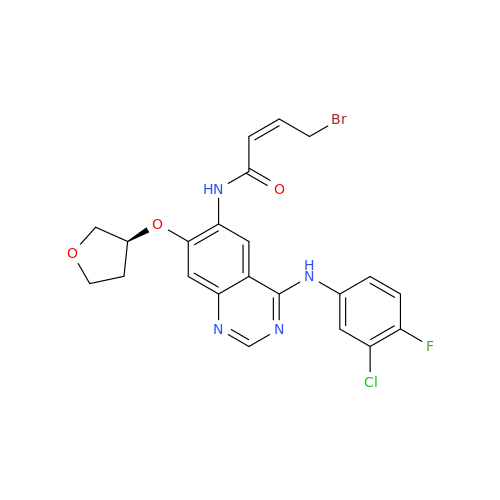
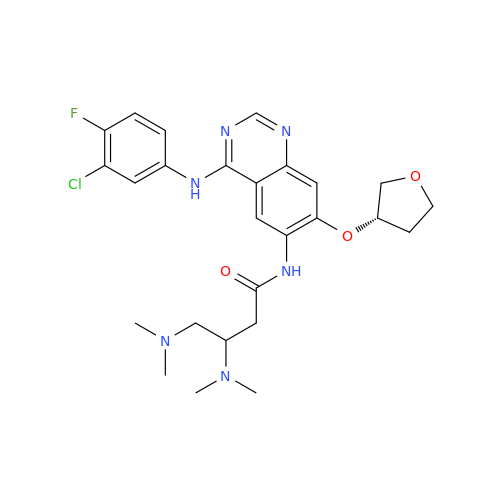
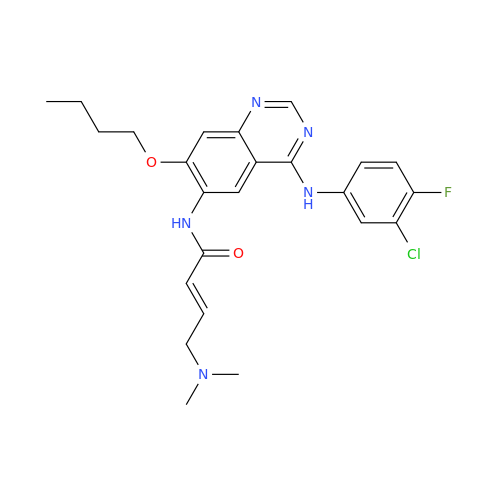
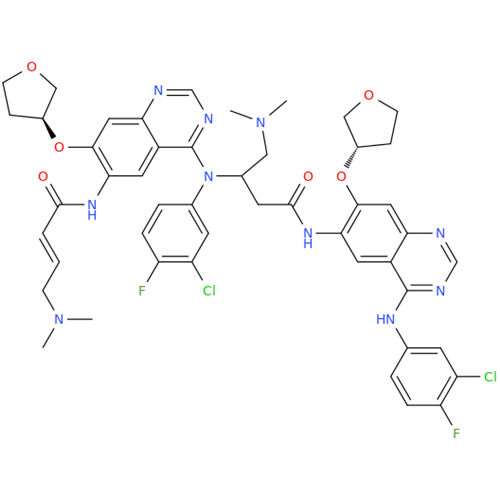
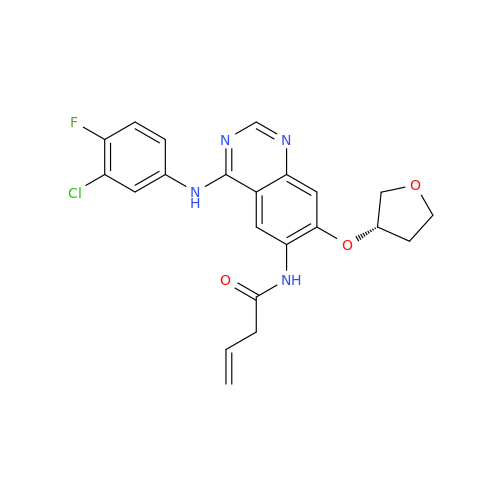
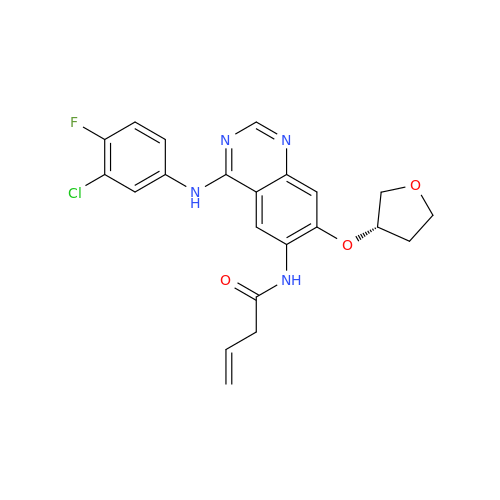
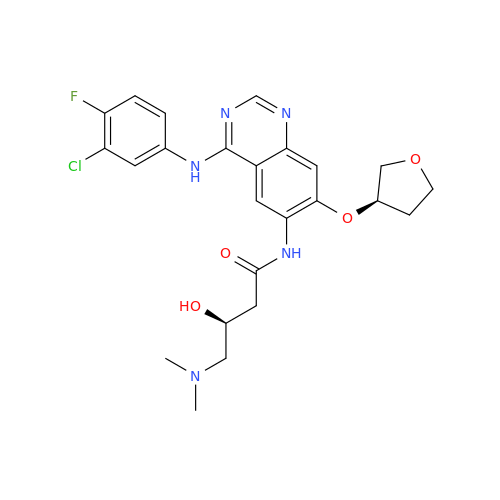
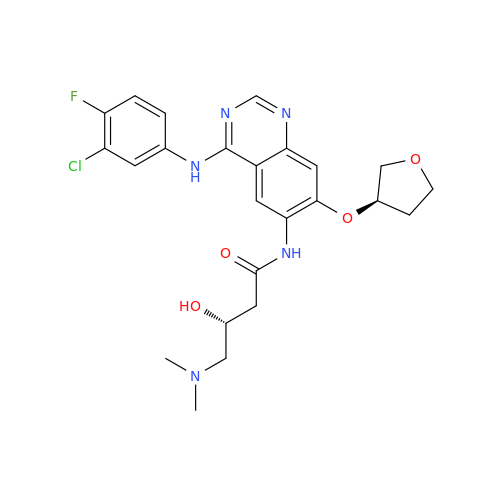
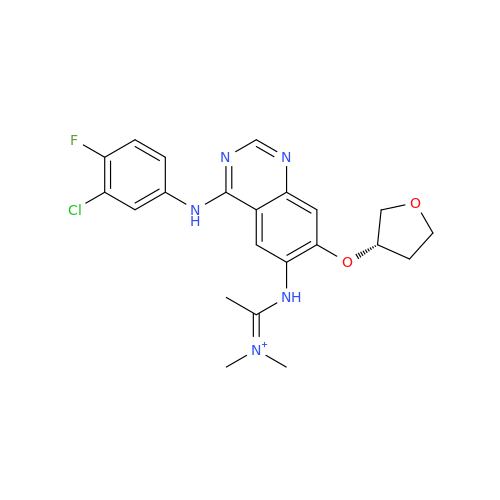
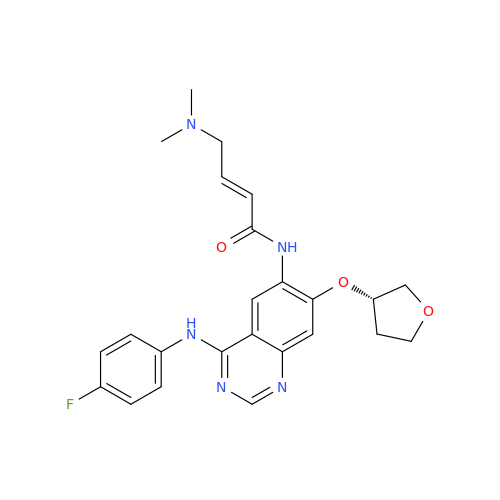
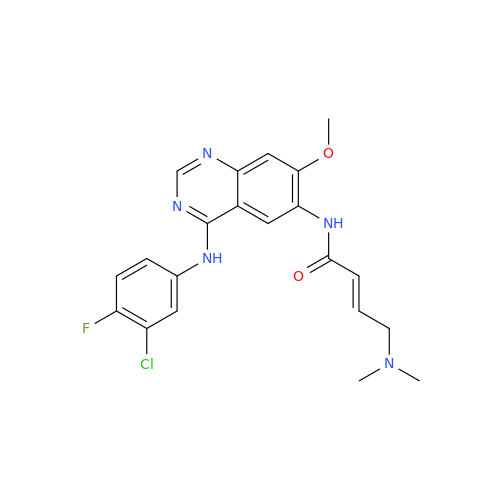
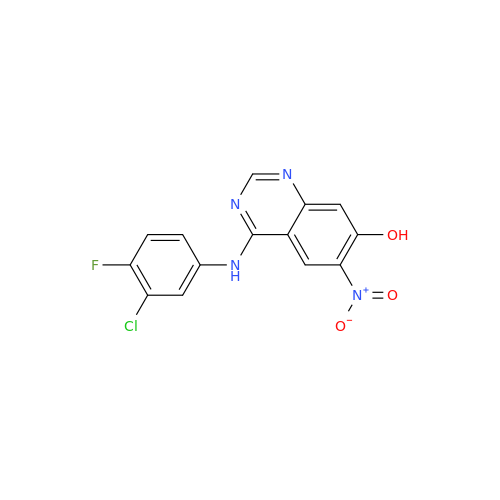
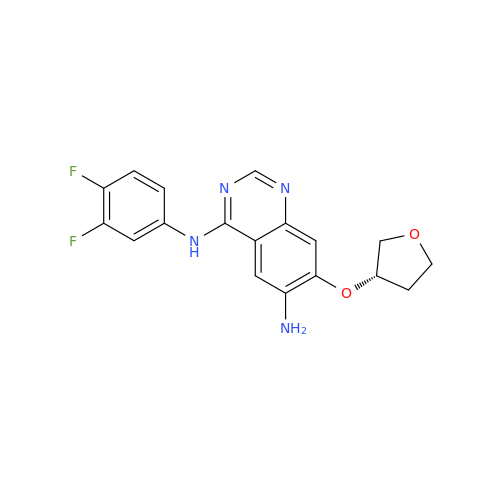
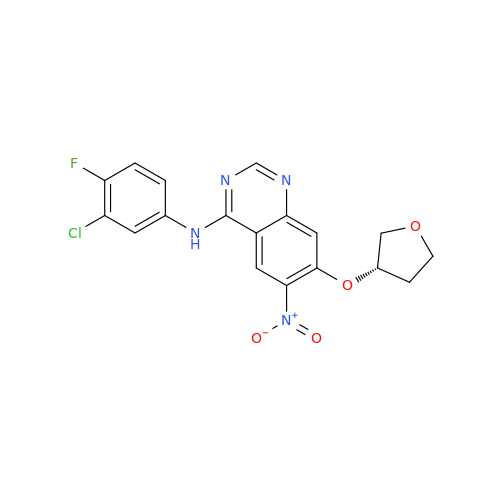
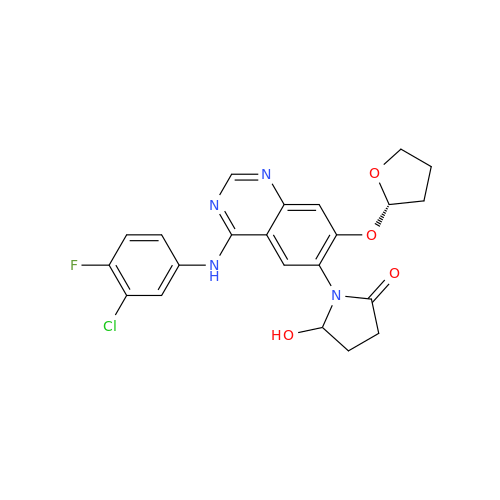
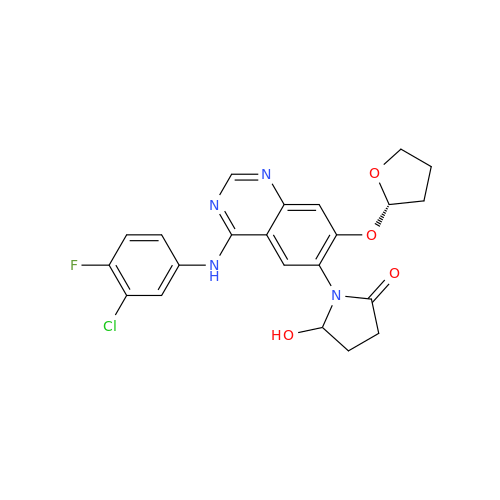
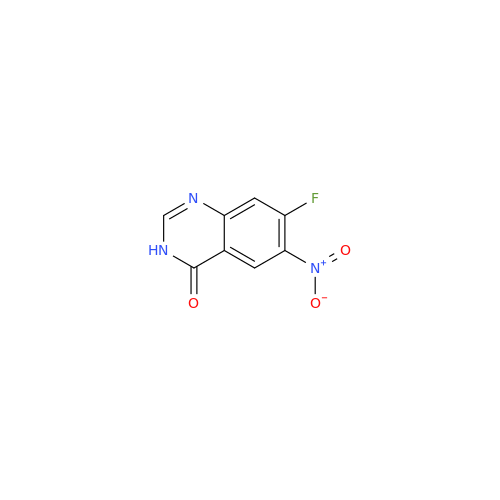
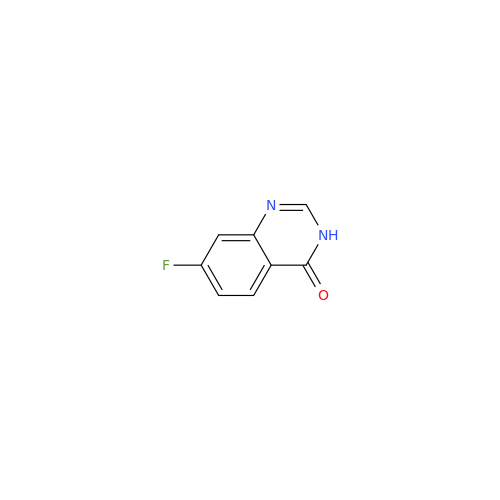
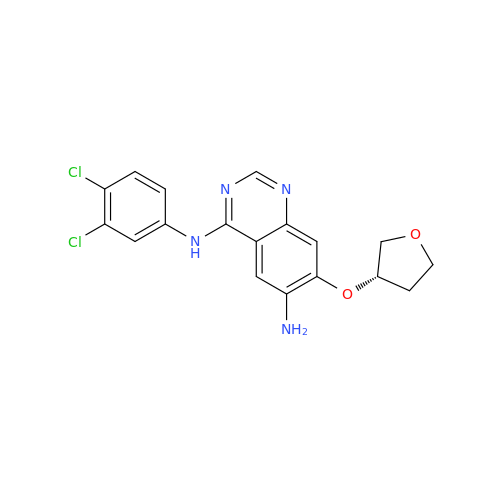
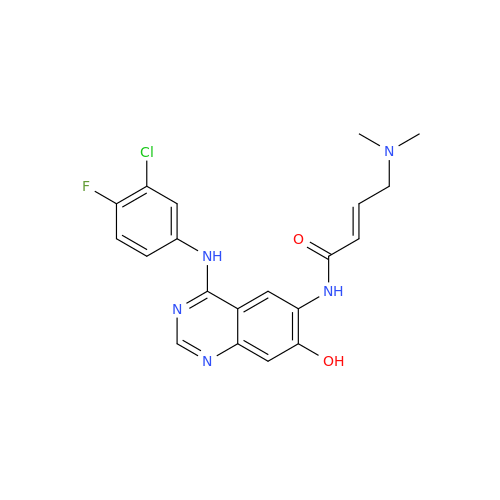
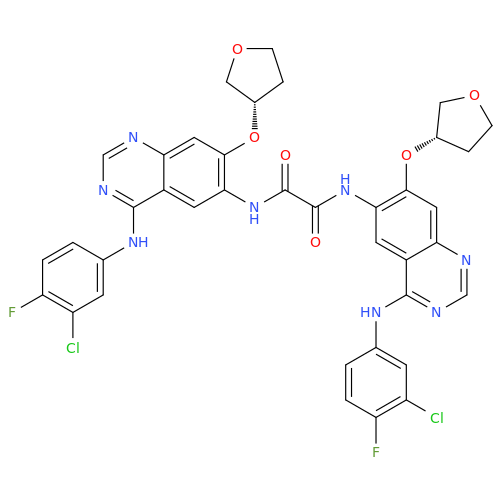
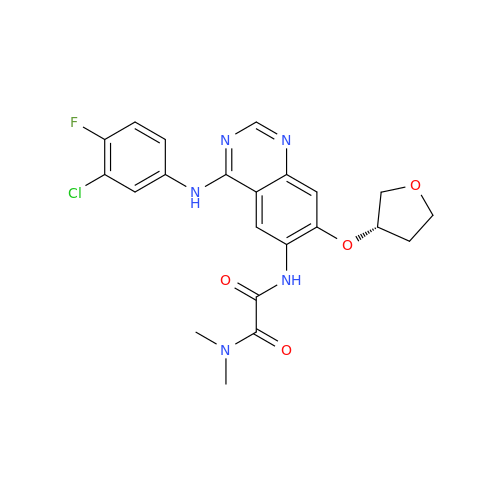
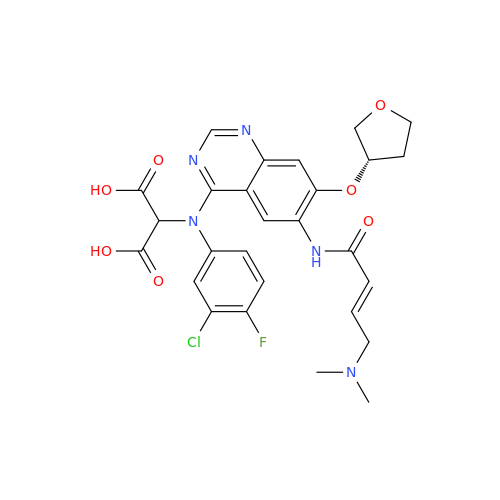
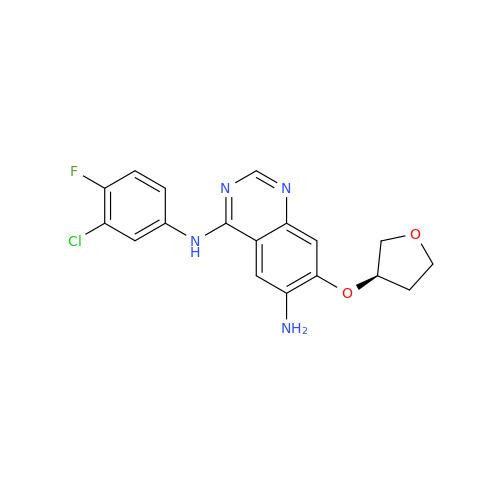
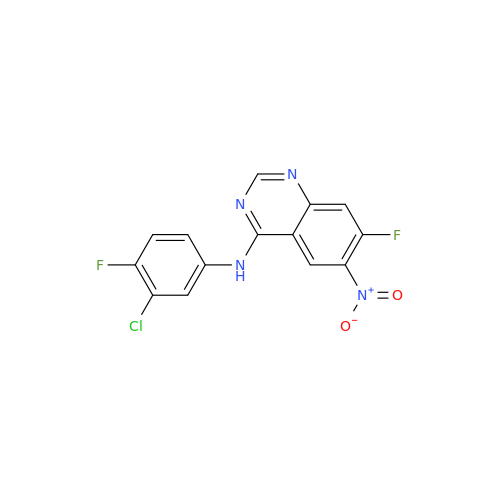
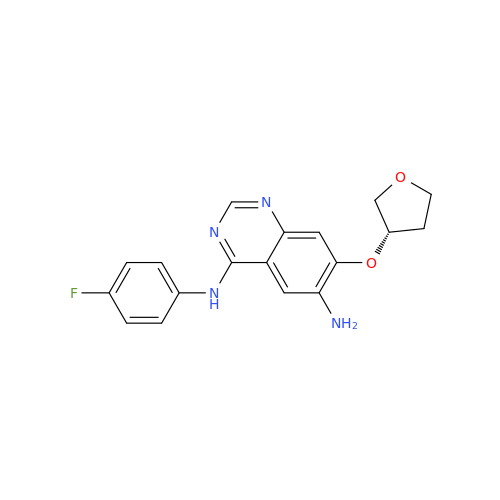
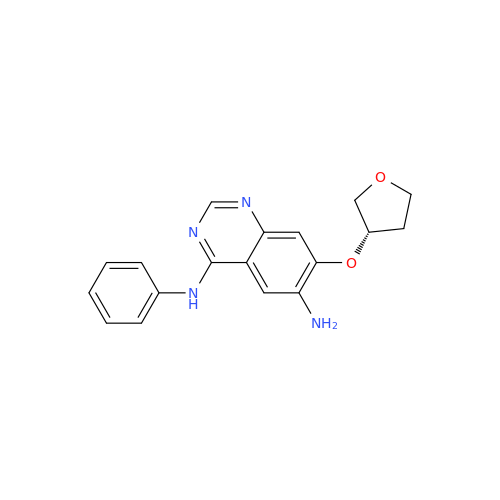
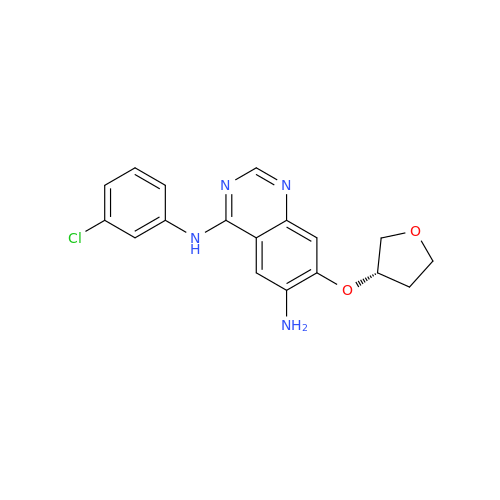
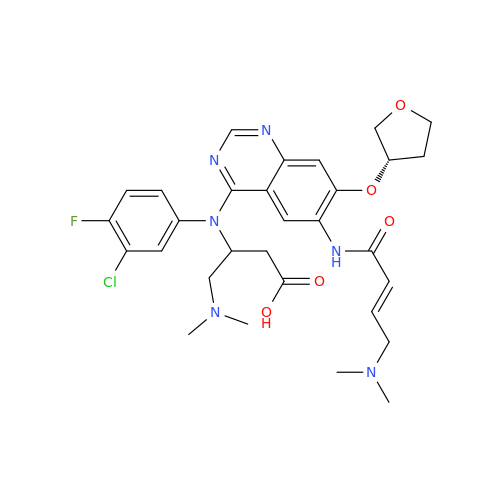
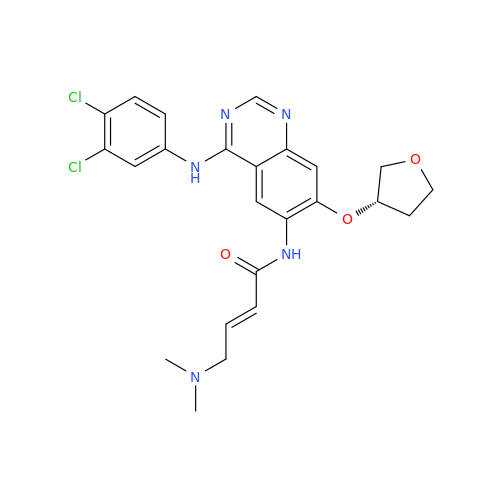
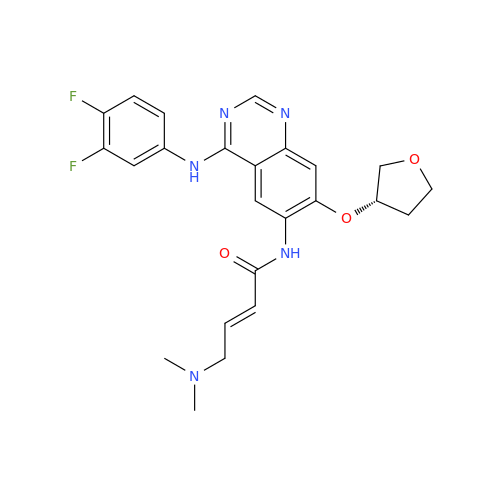
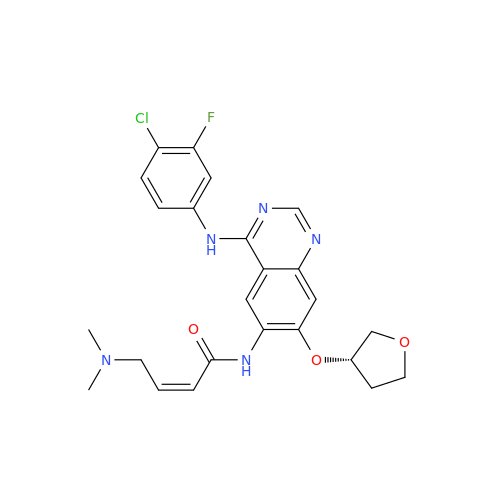
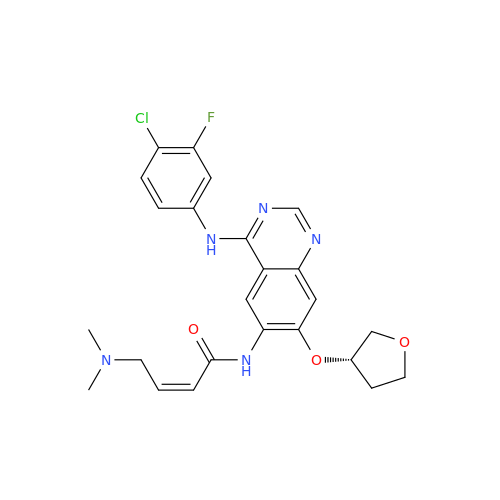
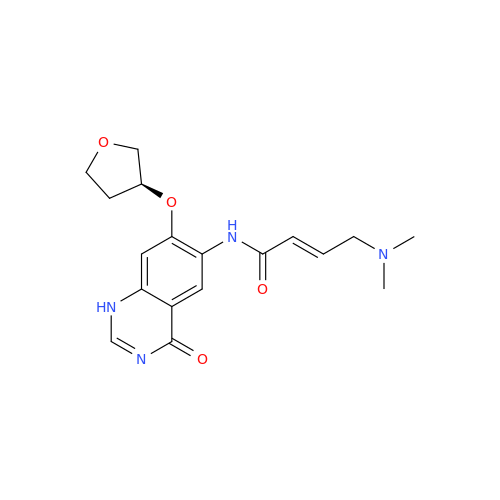
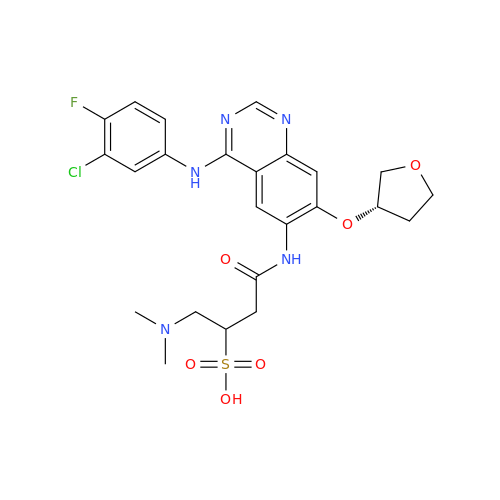
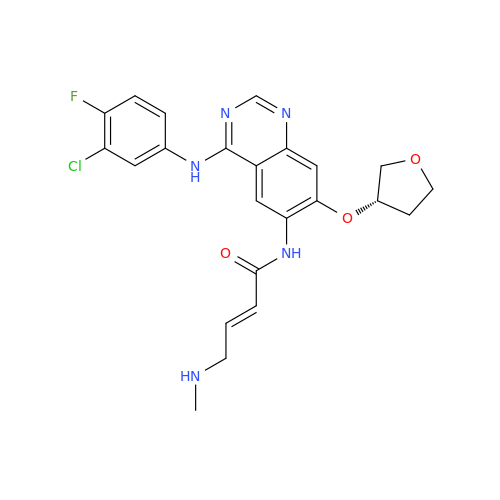
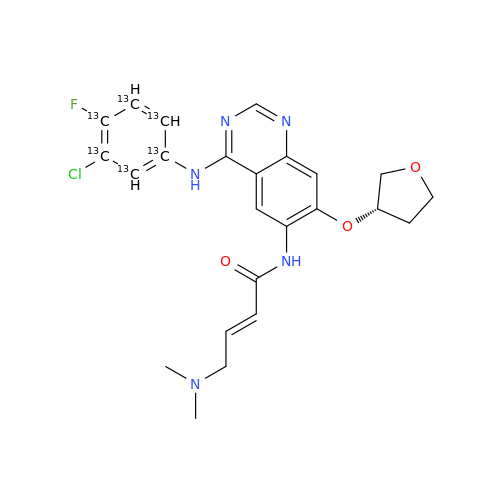
Afatinib Impurity 16
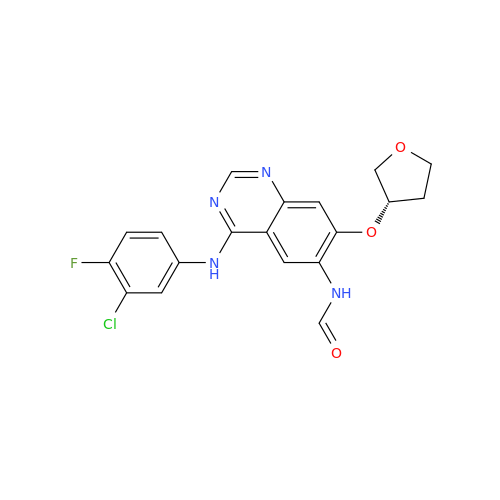
|
Chemical Name: Afatinib Impurity 16
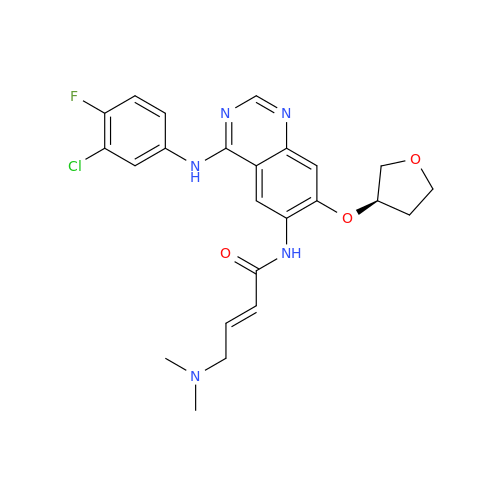
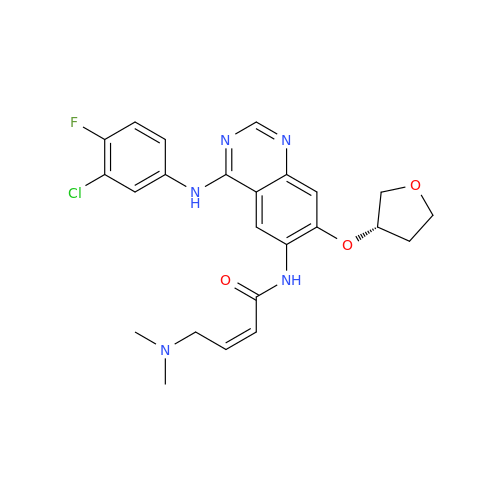
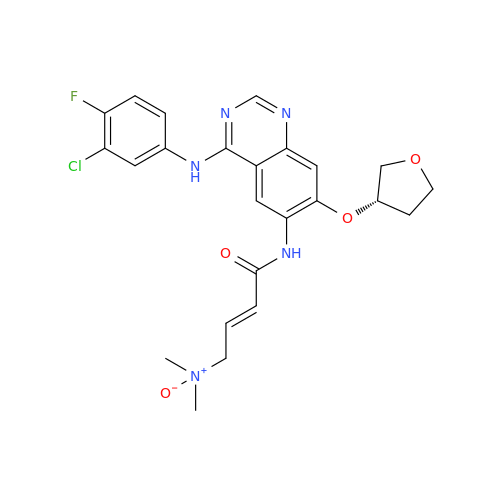
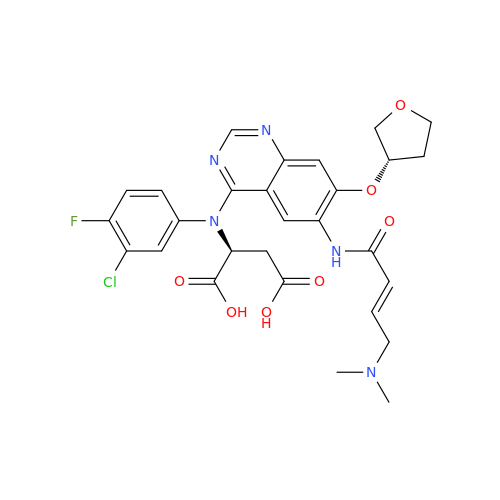
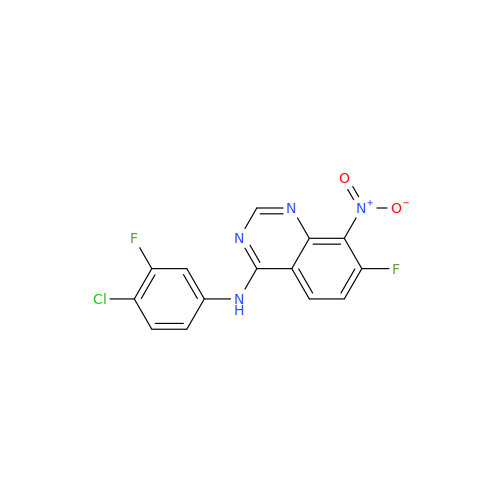
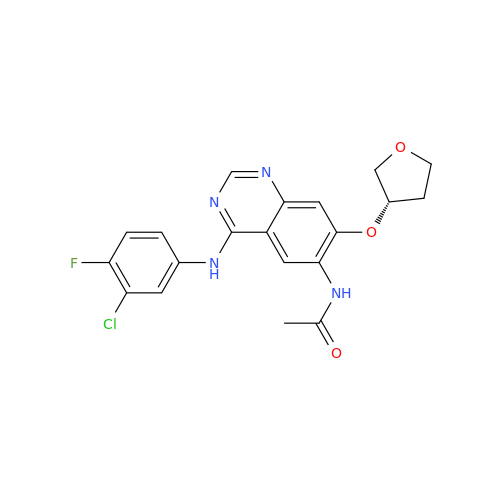
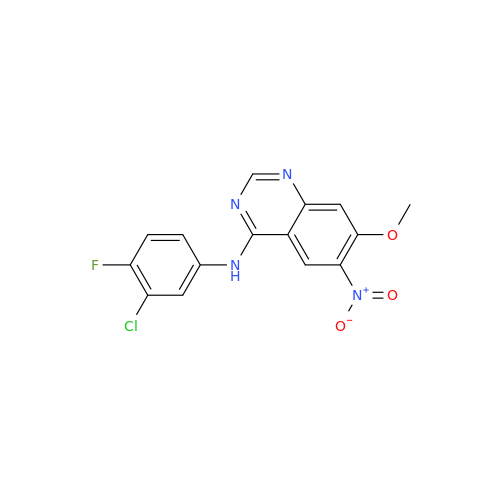
Synonym: Des(N,N-Dimethylprop-2-enyl-1-Amine) Afatinib; Des(4-dimethylamino-2-en-1-oxo)butyl Afatinib N6 Formyl| Enter Batch Number | |||