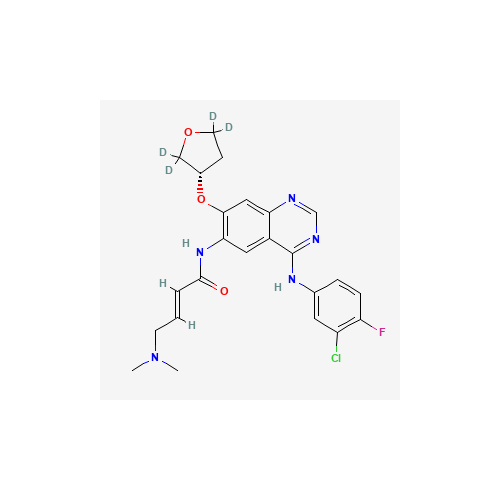
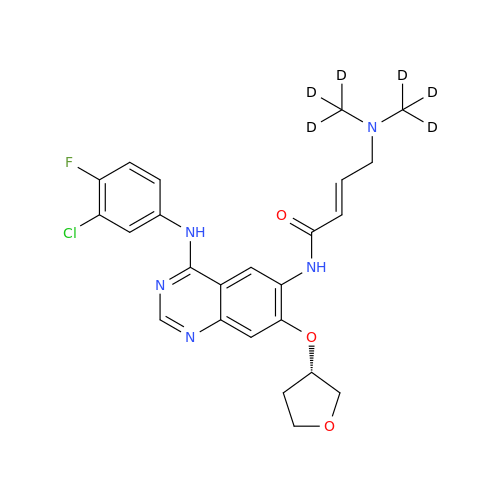
Product Information
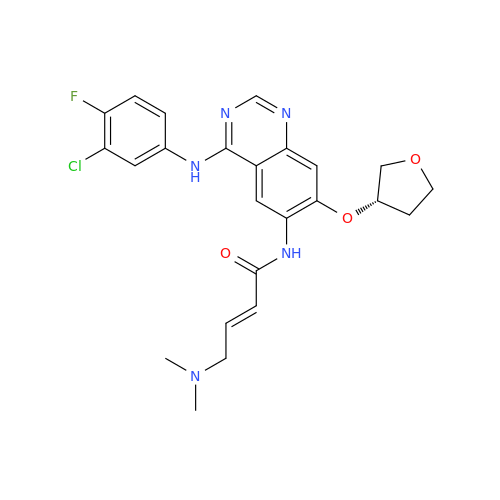
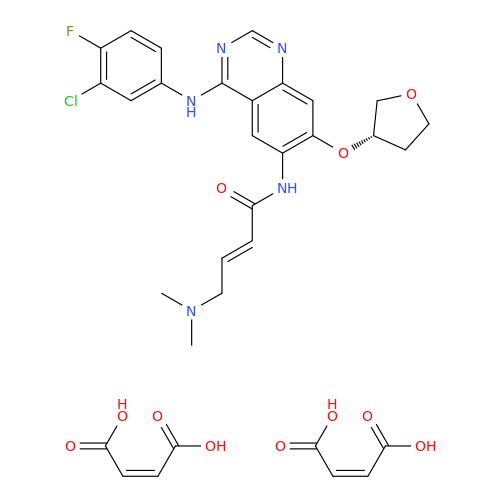
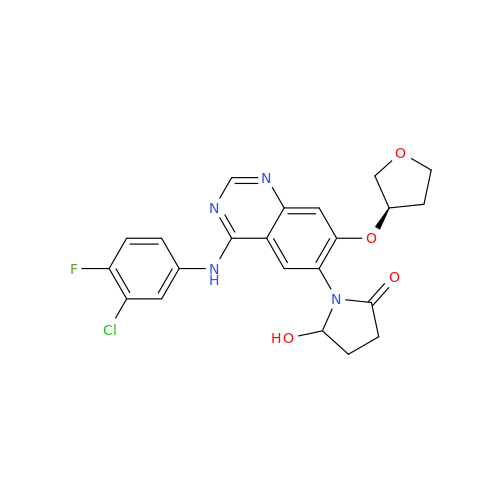
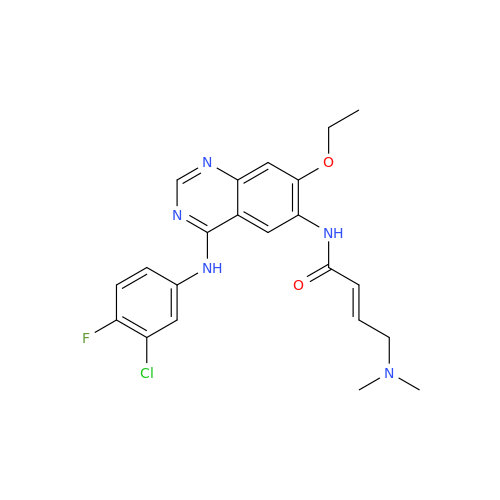
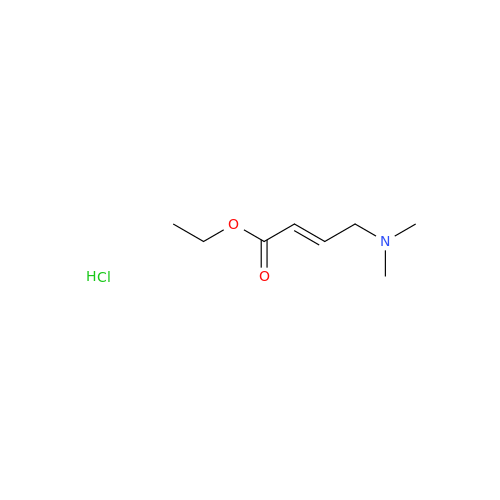
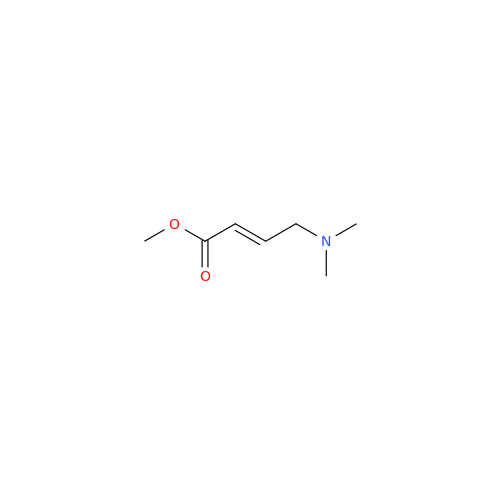
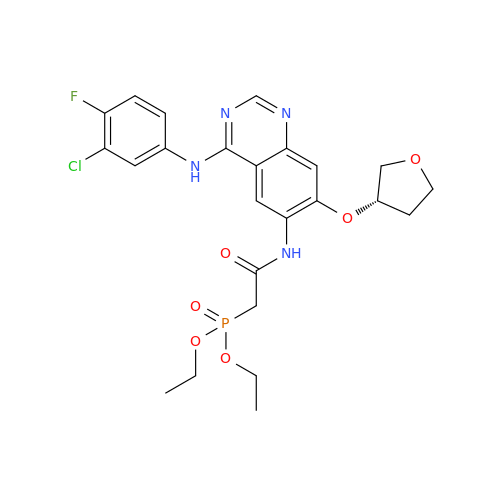
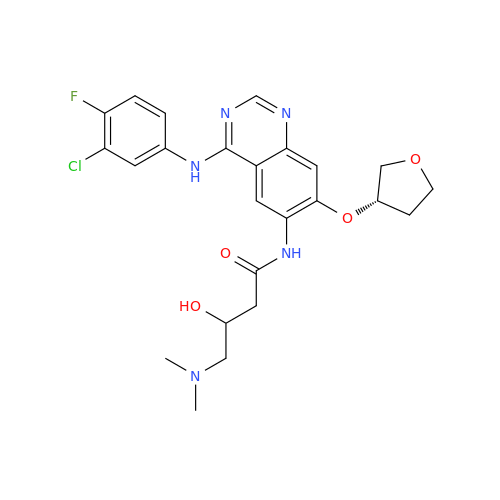
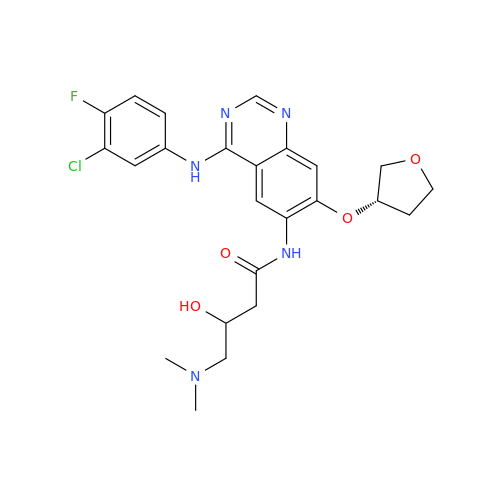
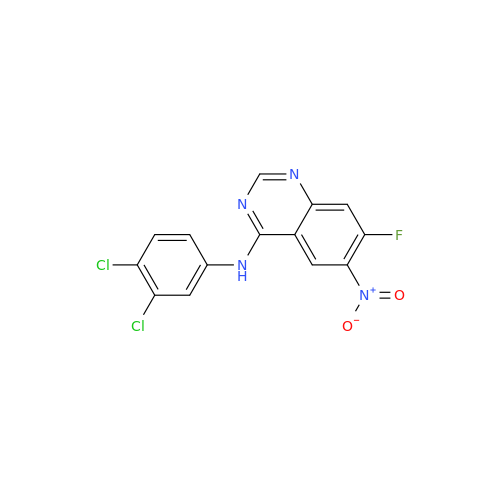
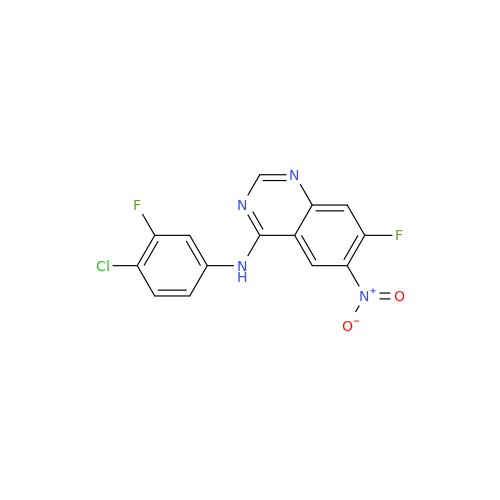
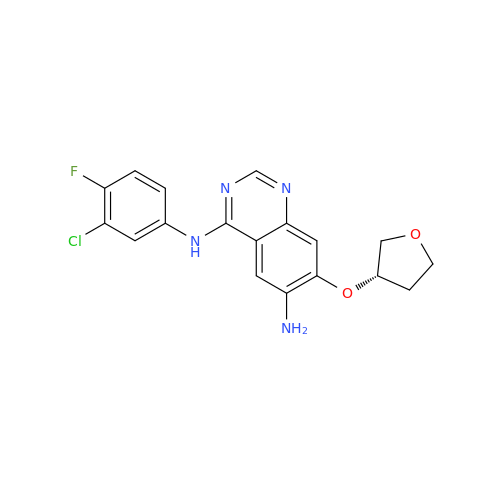
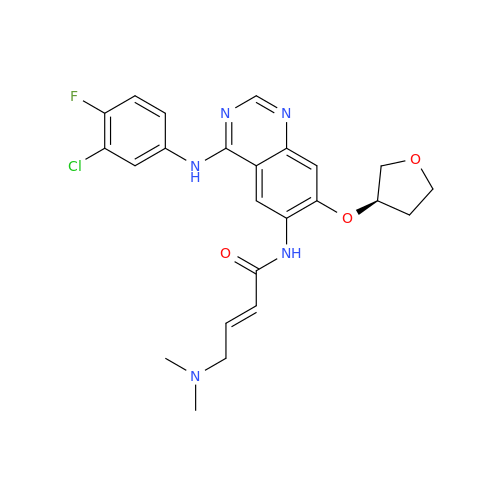
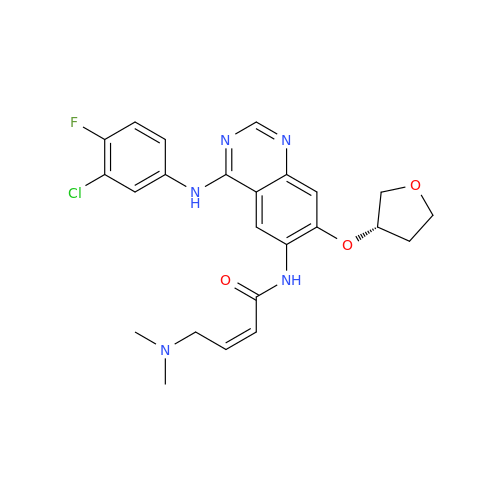
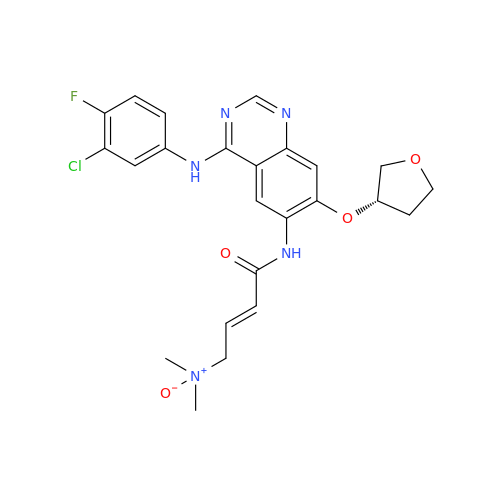
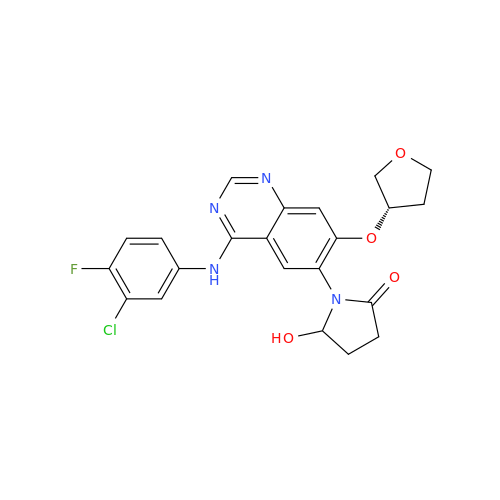
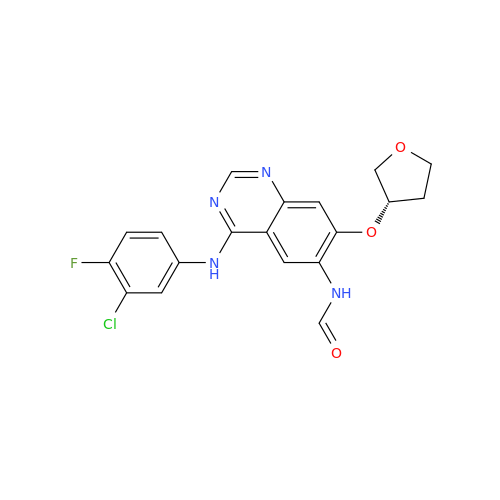
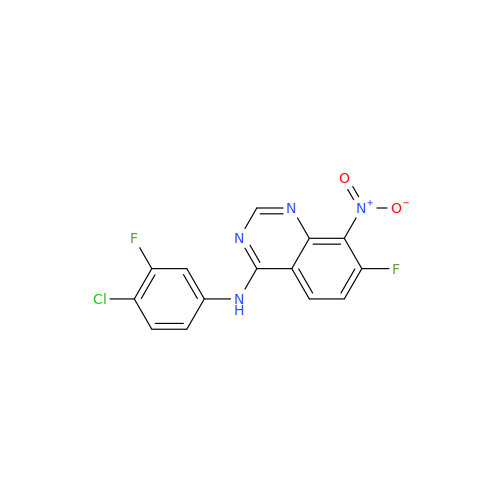
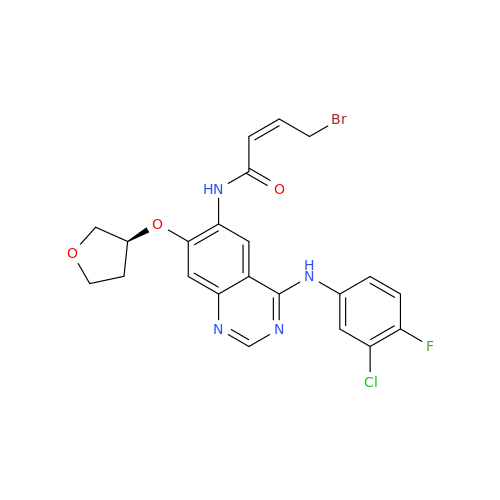
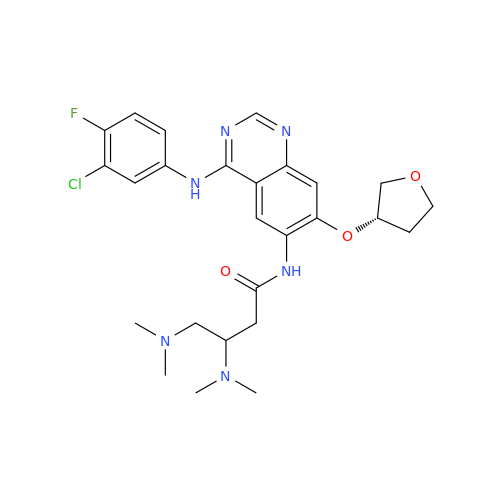
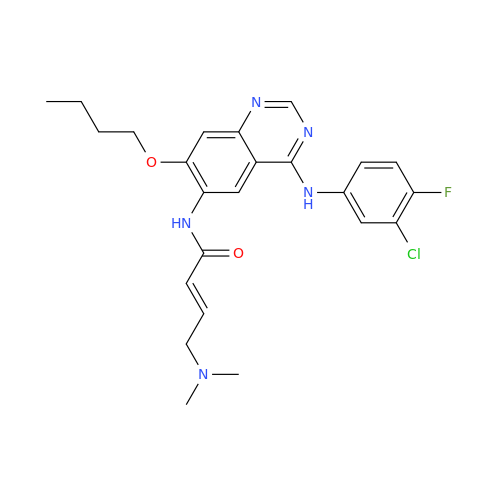
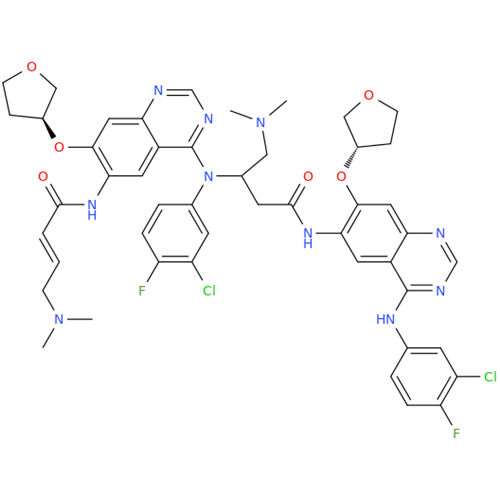
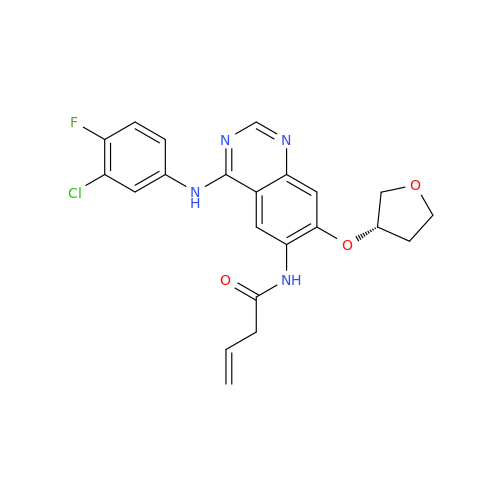
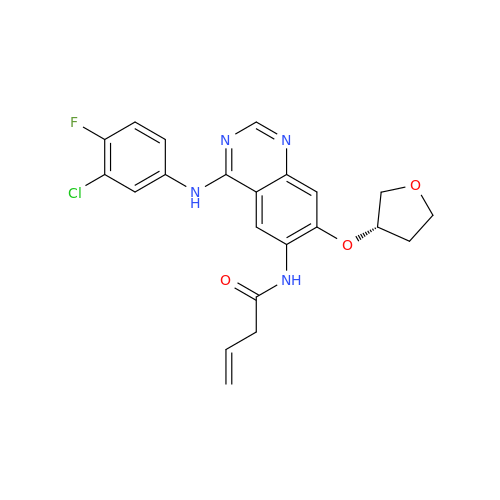
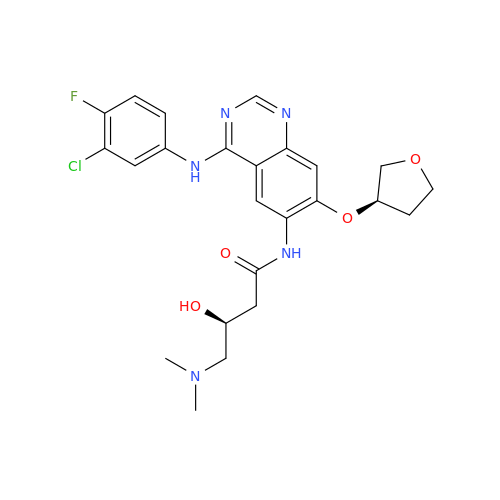
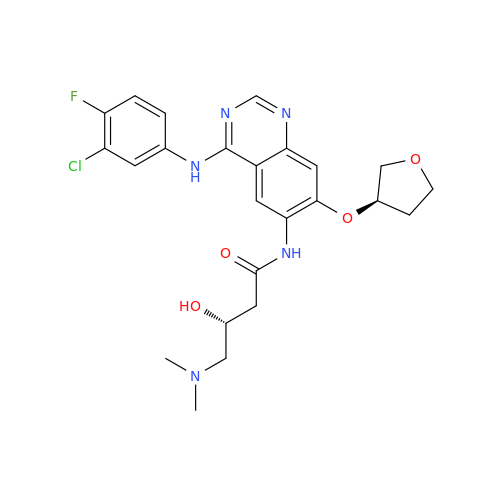
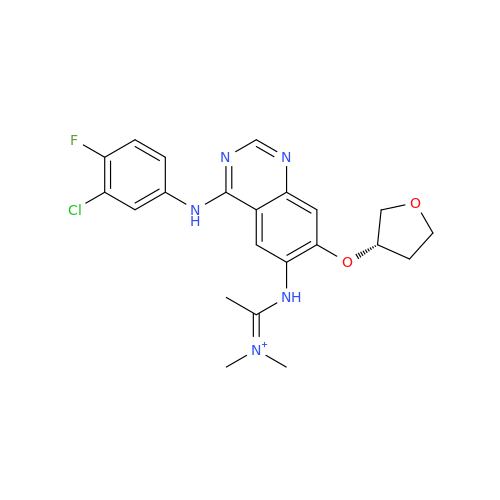
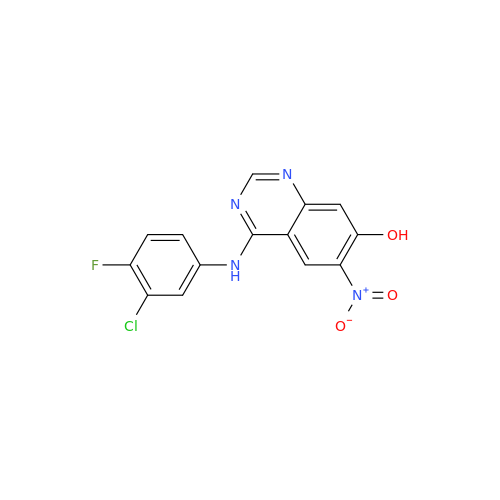
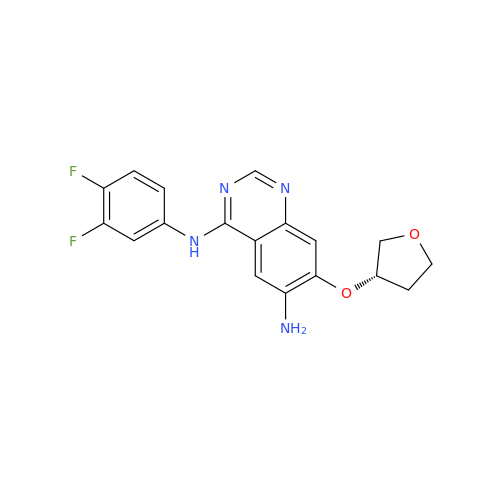
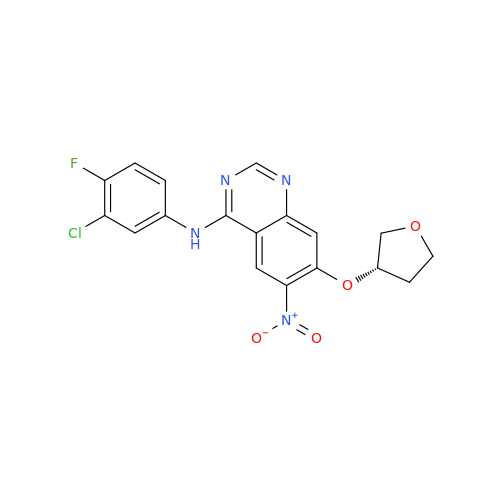
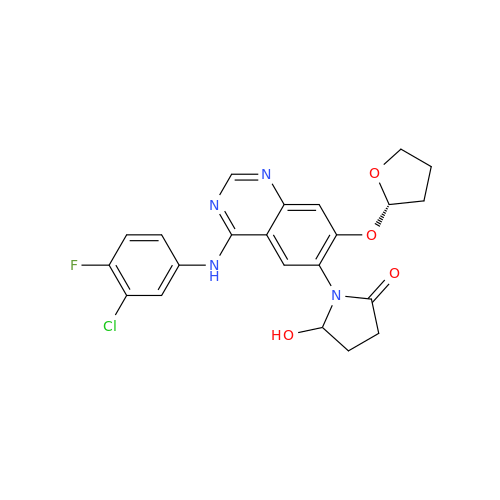
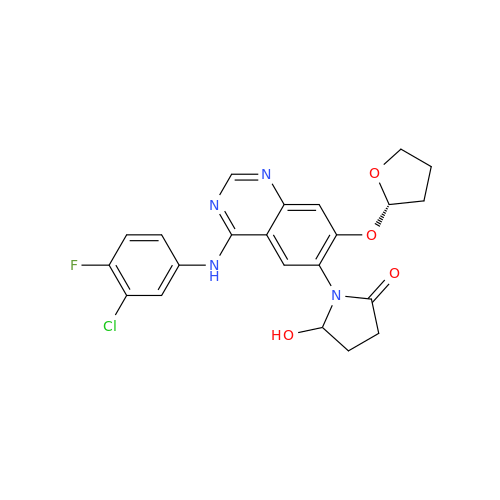
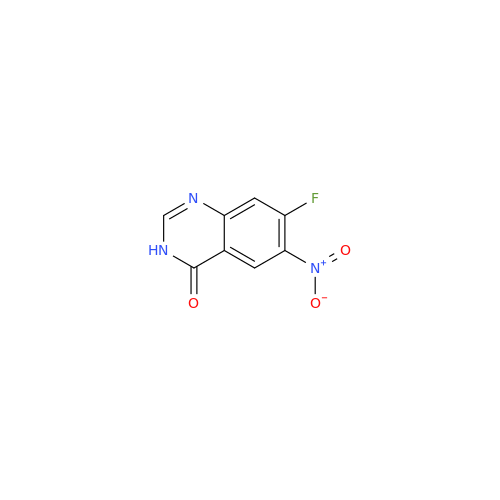
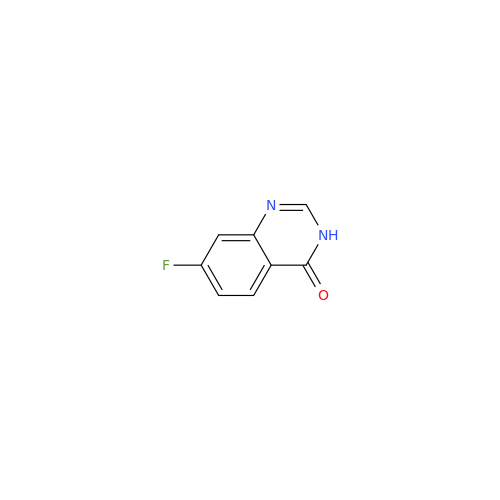
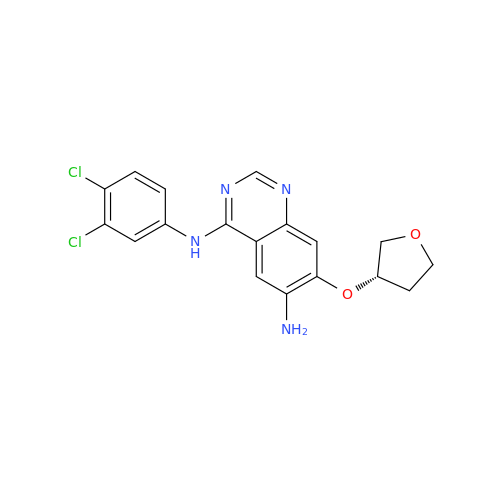
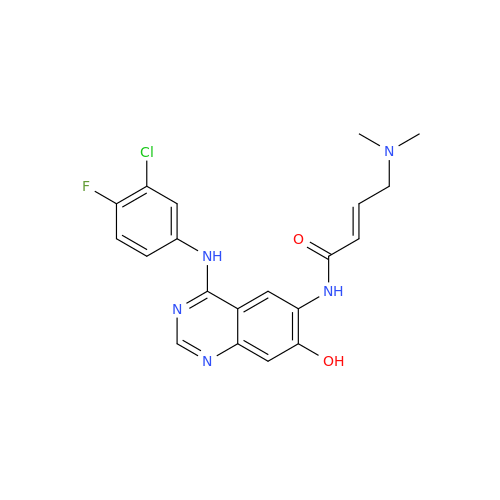
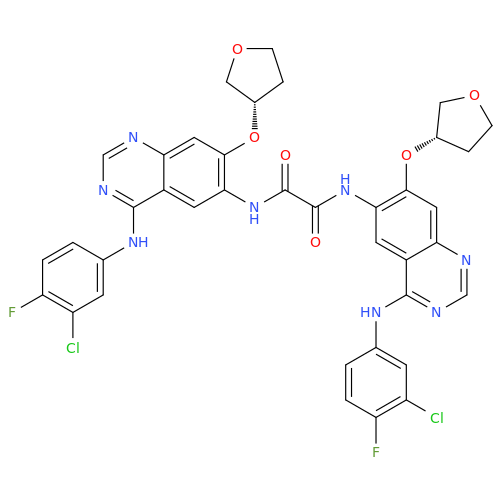
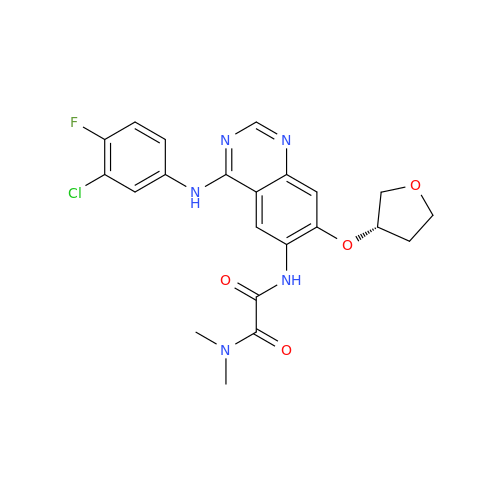
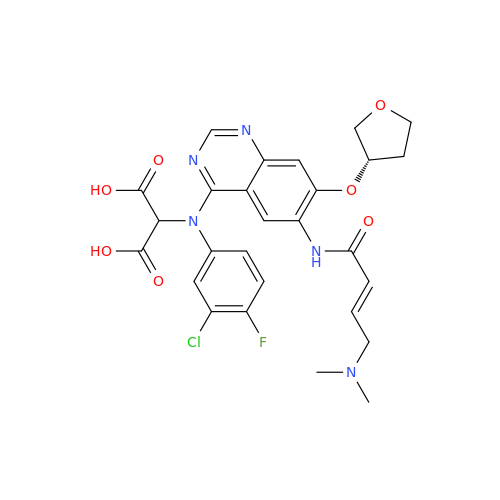
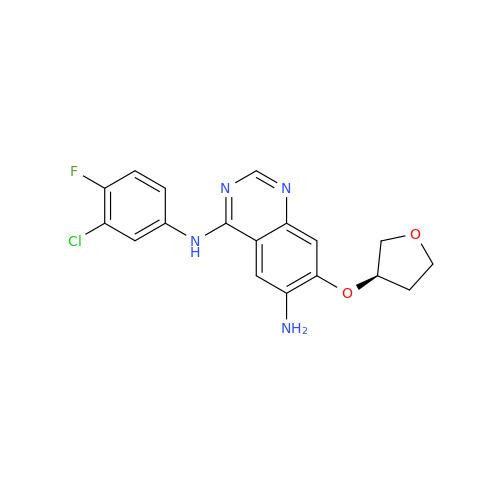
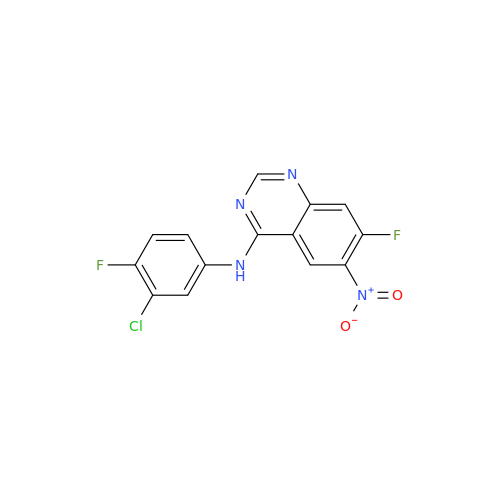
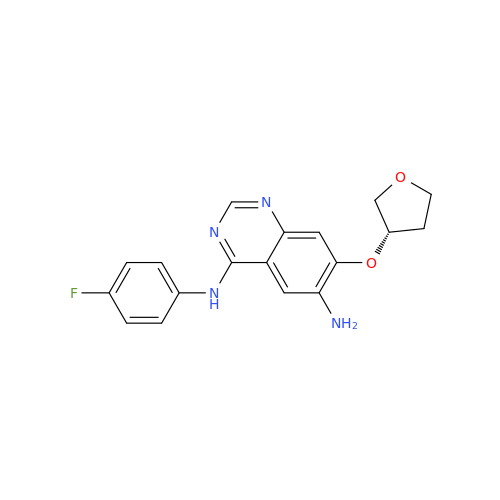
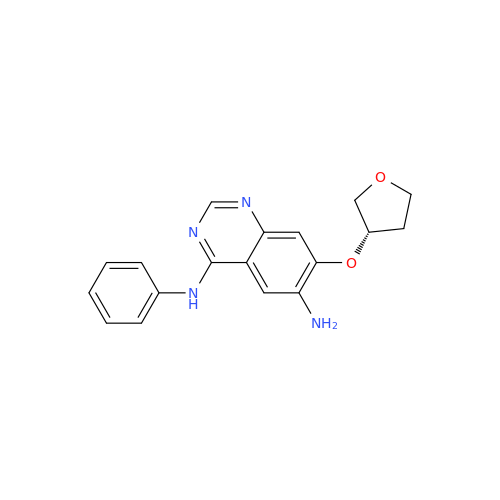
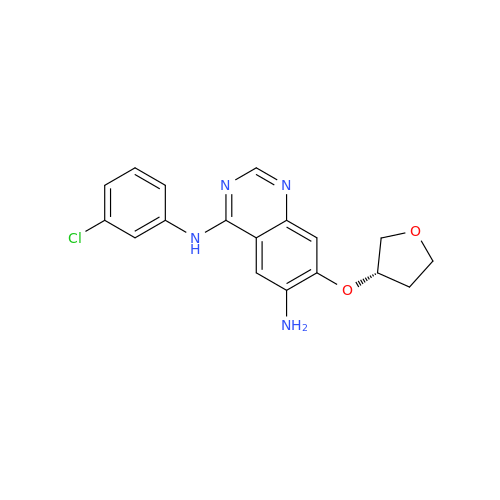
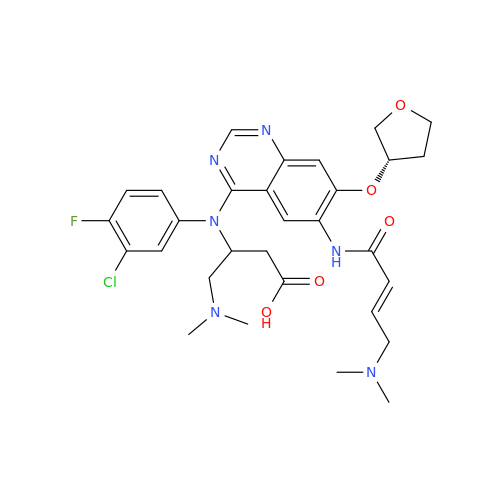
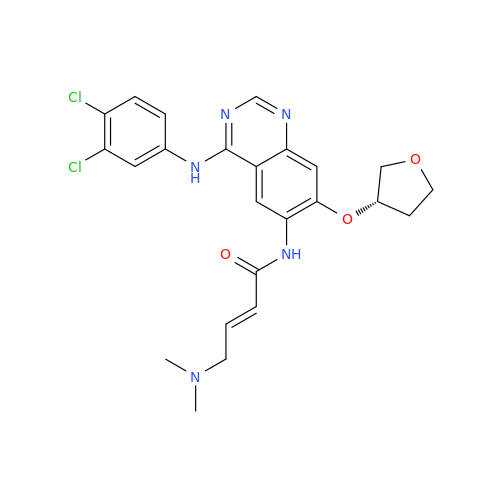
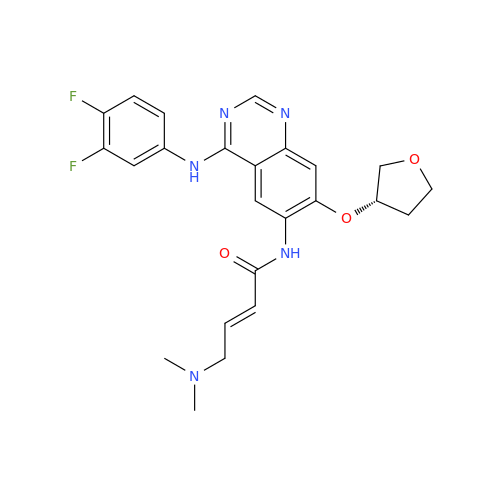
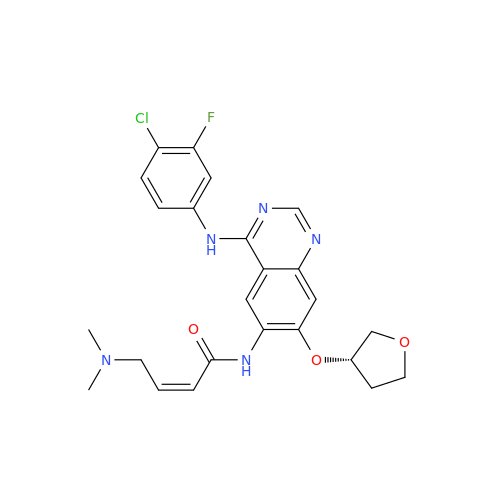
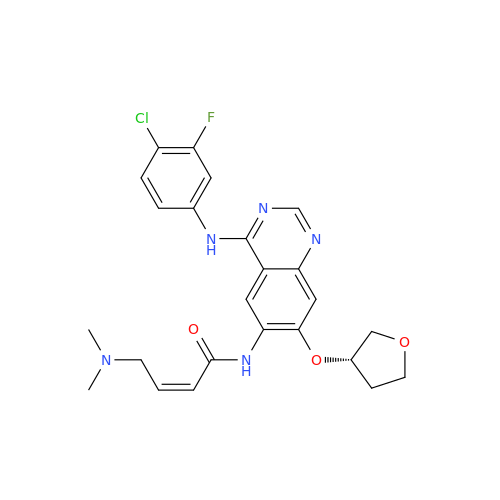
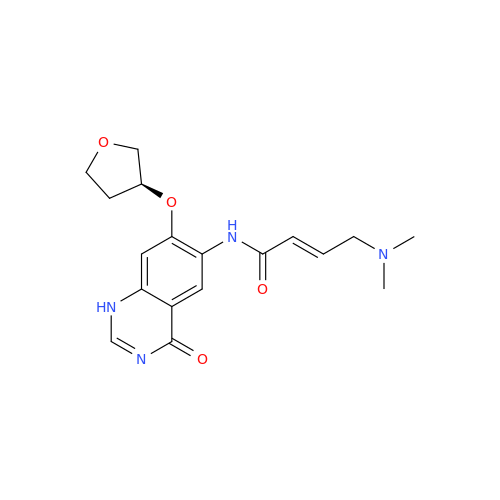
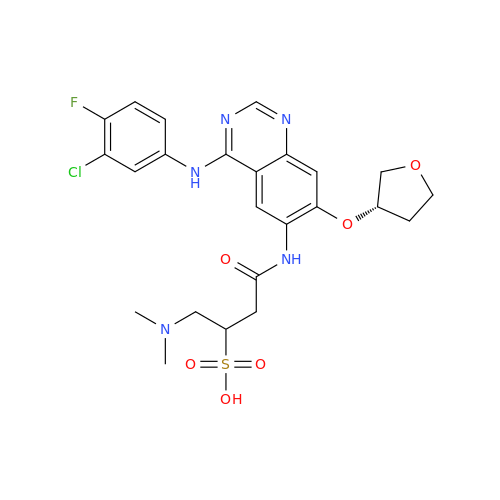
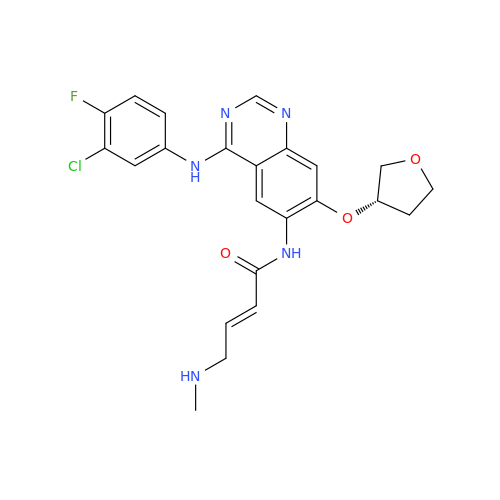
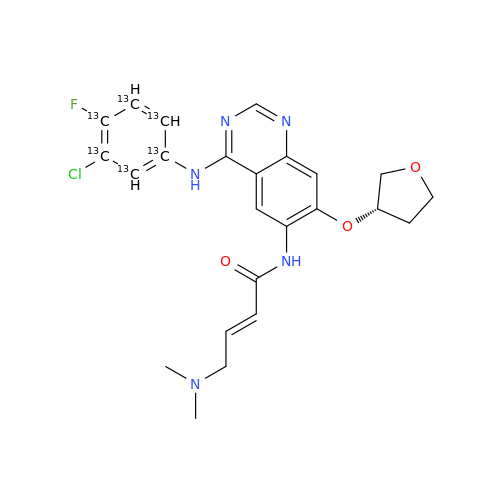
Afatinib Impurity A
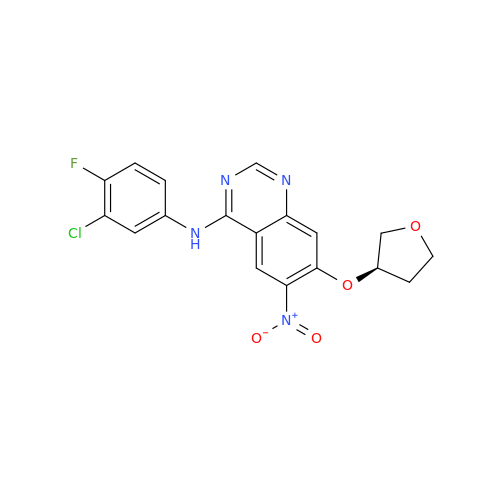
|
Chemical Name: Afatinib Impurity A
Synonym: (R)-N-(3-Chloro-4-fluorophenyl)-6-nitro-7-((tetrahydrofuran-3-yl)oxy)quinazolin-4-amine| Enter Batch Number | |||