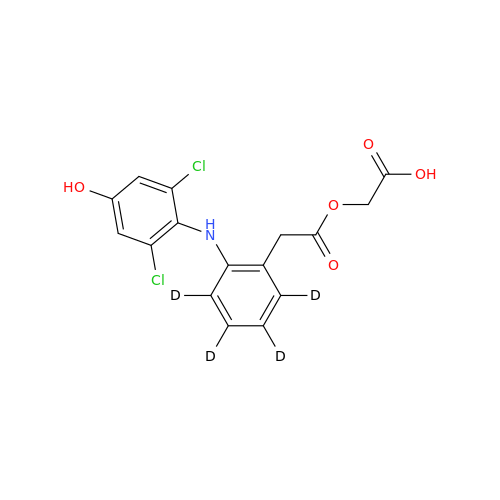
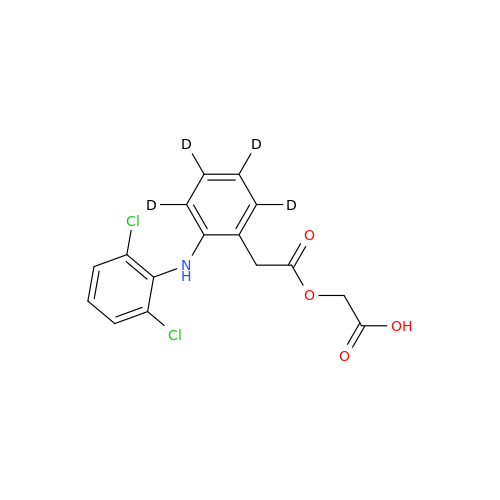
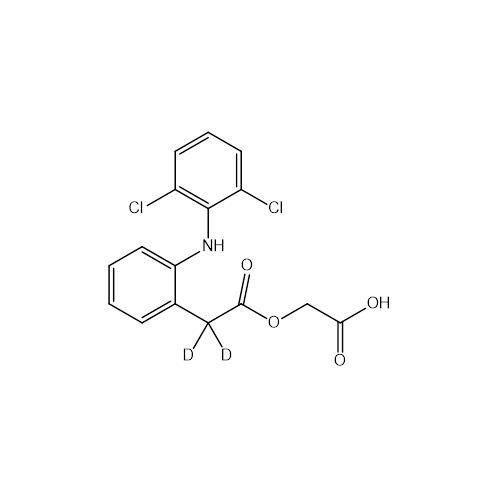
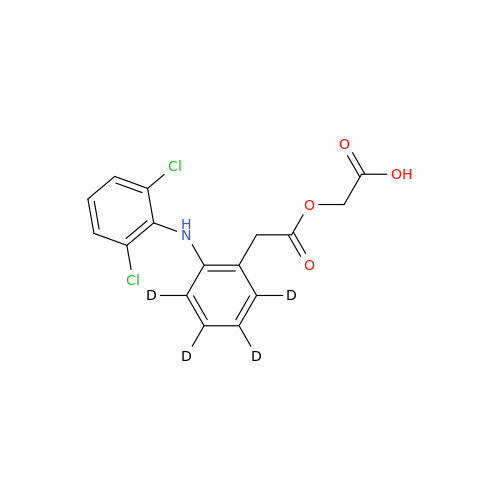
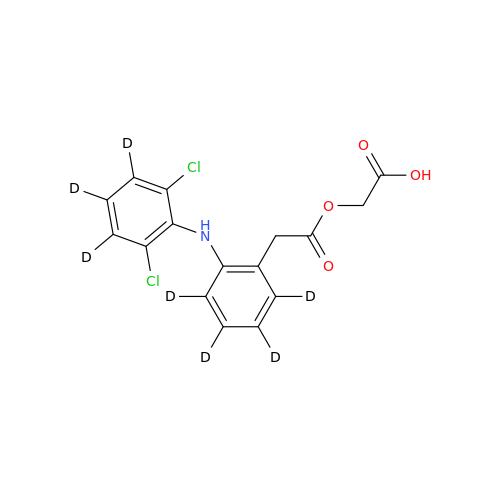
Product Information
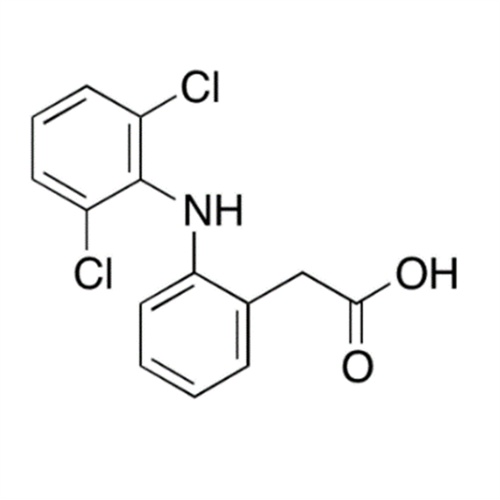
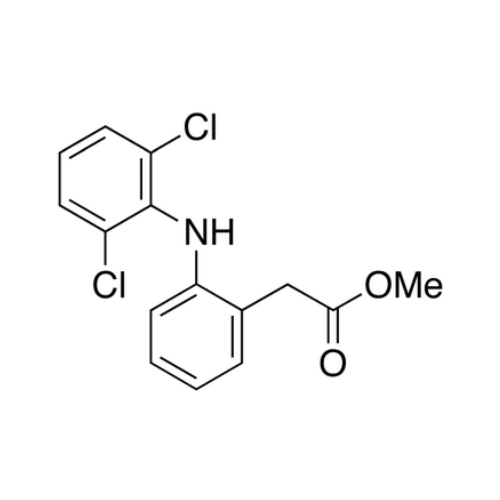
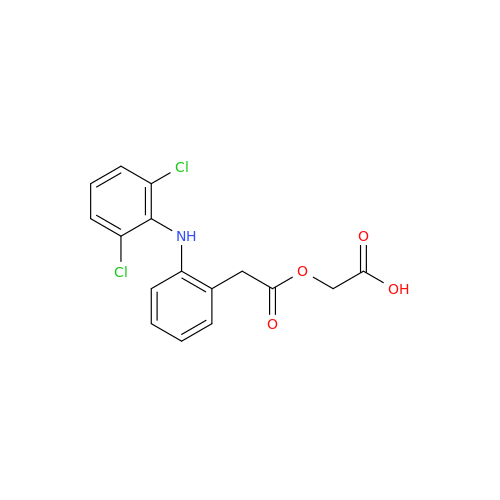
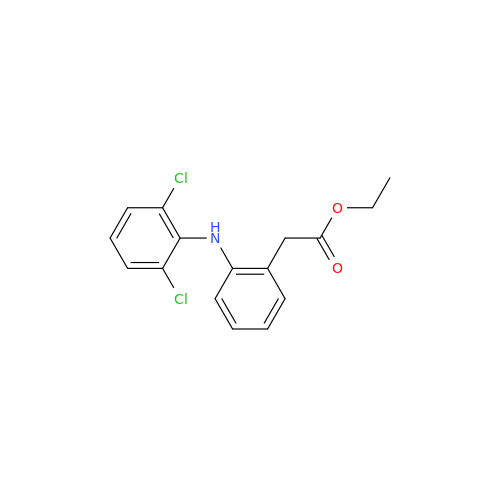
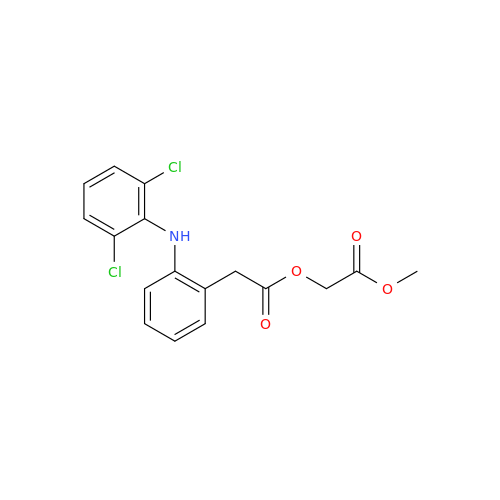
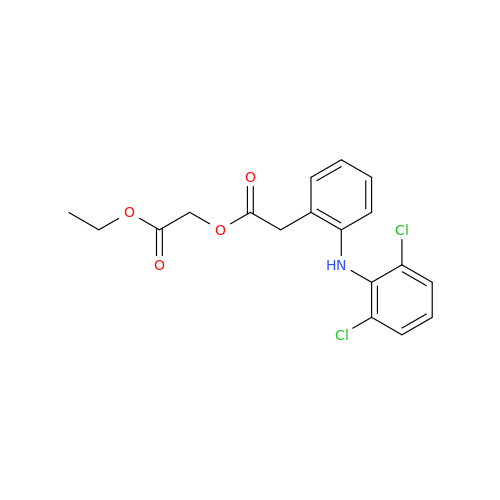
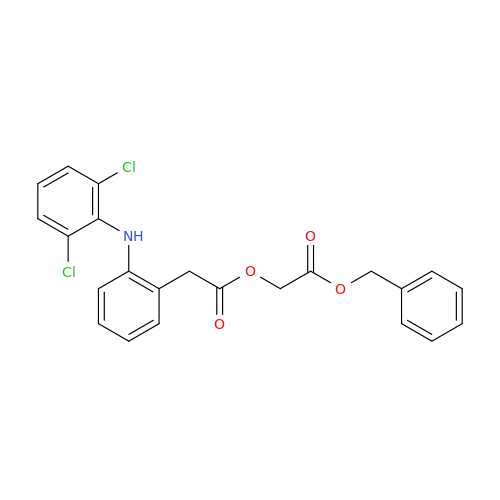
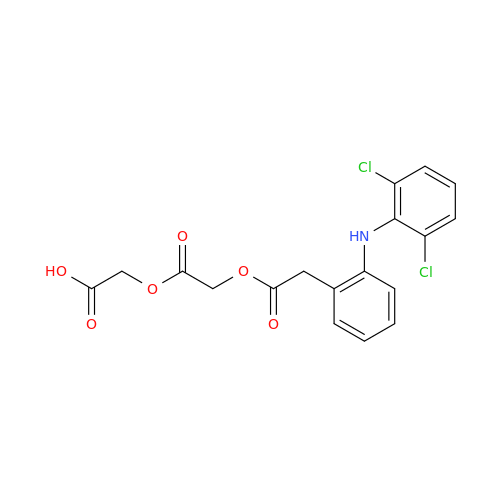
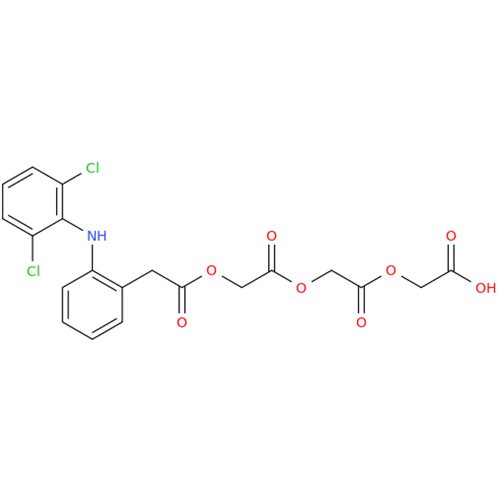
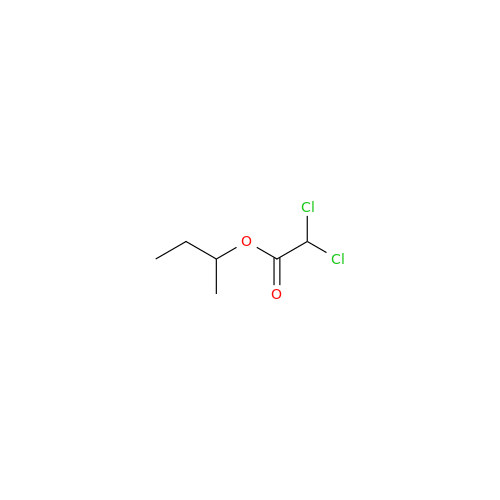
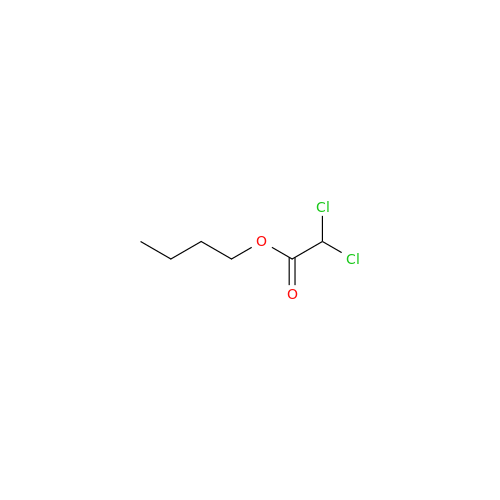
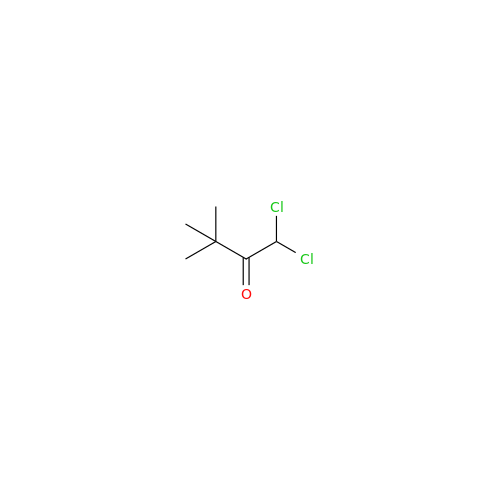
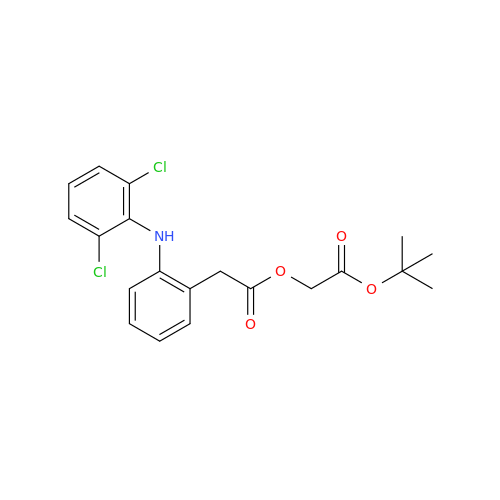
Aceclofenac EP Impurity I
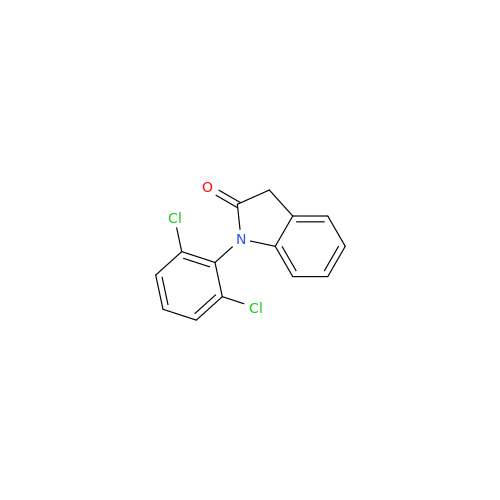
|
Chemical Name: Aceclofenac EP Impurity I
Synonym: Diclofenac Lactam; Diclofenac Amide; 1-(2,6-dichlorophenyl)indolin-2-one; 1-(2,6-Dichlorophenyl)-1,3-dihydro-2H-indol-2-one;1-(2,6-Dichlorophenyl)oxindole; N-(2,6-dichlorophenyl)-2-indolinone; Diclofenac Impurity A| Enter Batch Number | |||