Product Information
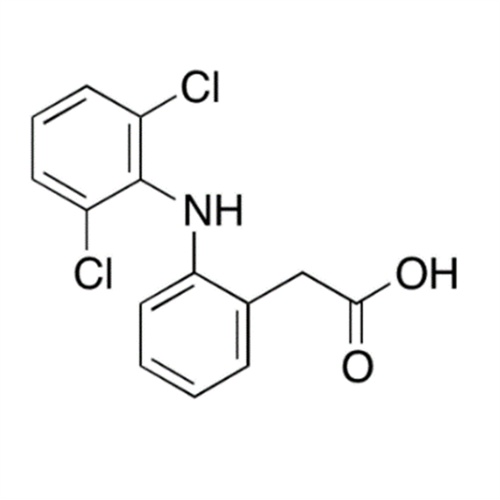
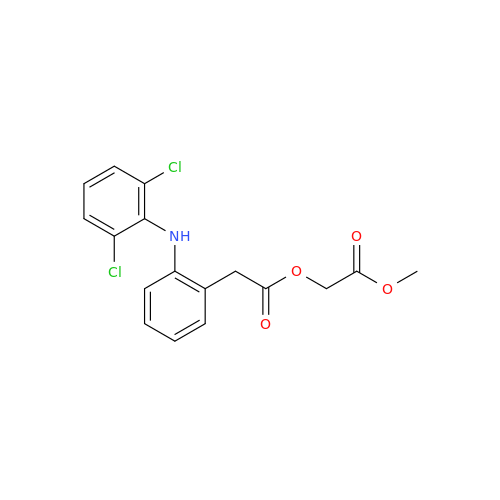
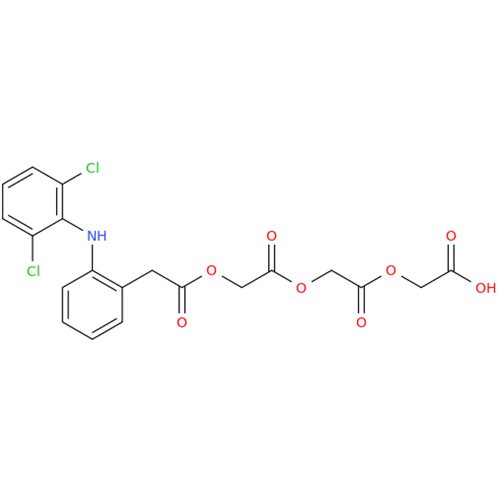
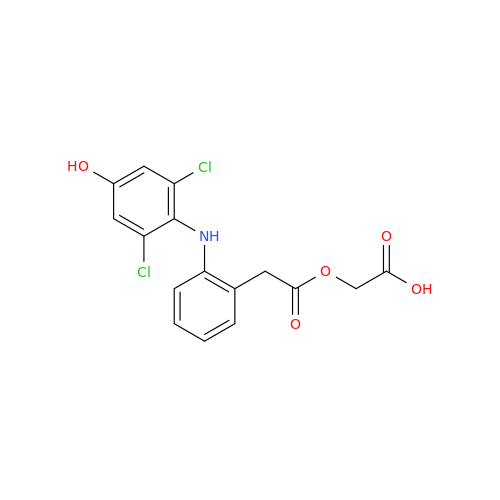
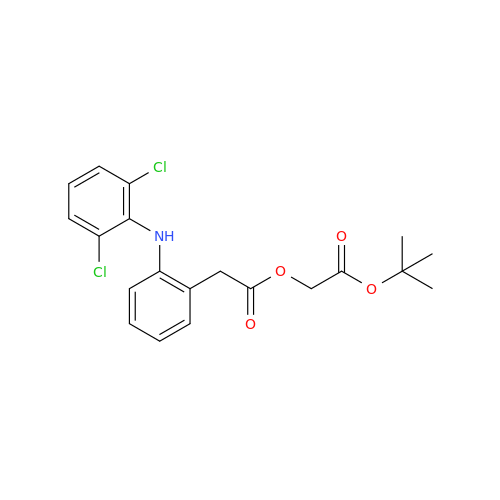
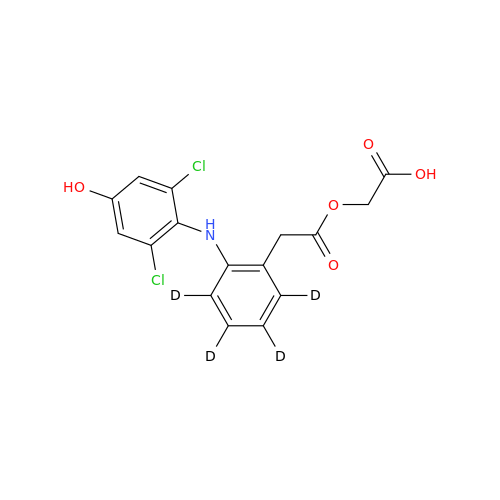
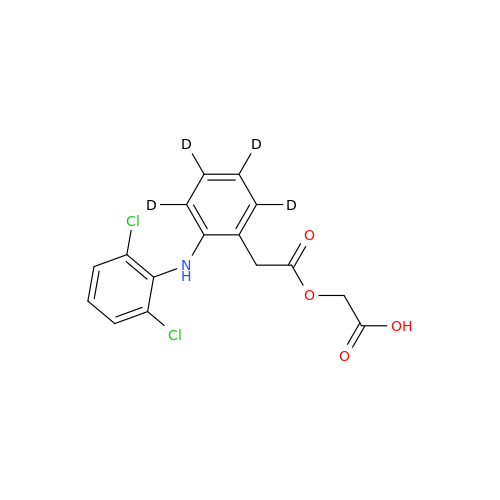
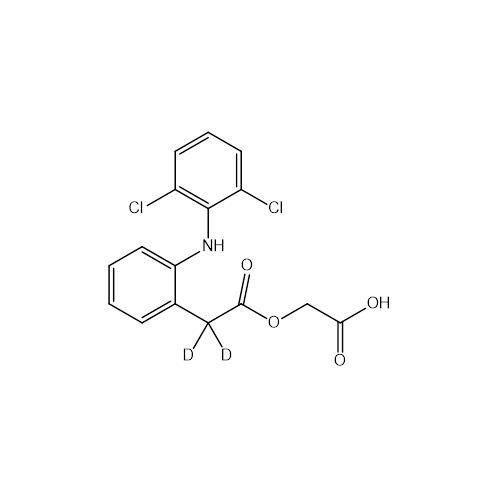
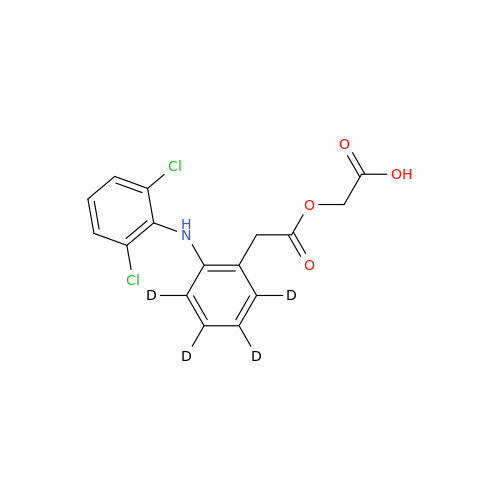
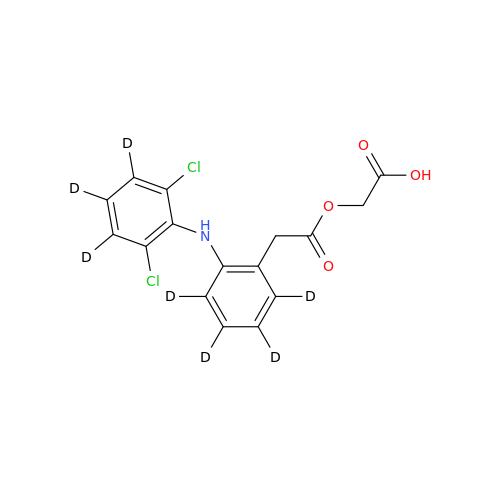
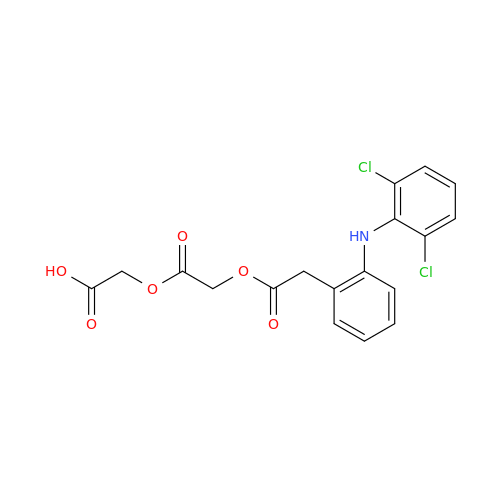
Aceclofenac EP Impurity G
|
Chemical Name: Aceclofenac EP Impurity G
Synonym: [[[[[2-[(2,6-Dichlorophenyl)amino]phenyl]acetyl]oxy]acetyl]oxy]acetic acid; 2-(2-(2-(2-((2,6-dichlorophenyl)amino)phenyl)acetoxy)acetoxy)acetic acid; 2-[(2,6-Dichlorophenyl)amino]benzeneacetic Acid 2-(Carboxymethoxy)-2-oxoethyl Ester| Enter Batch Number | |||