Product Information
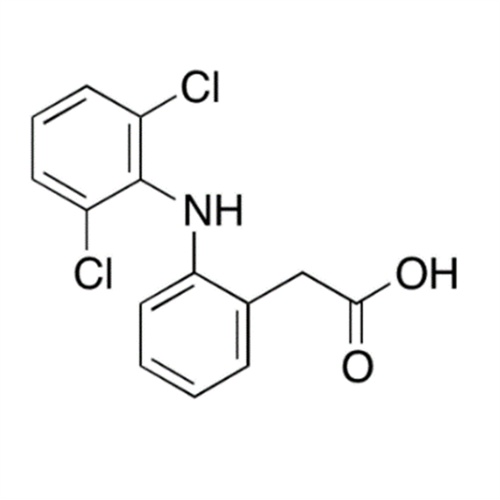
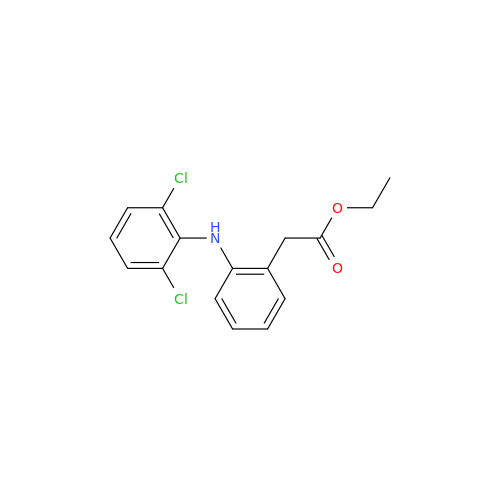
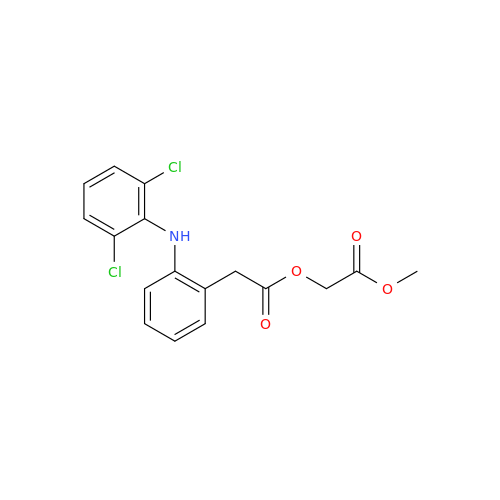
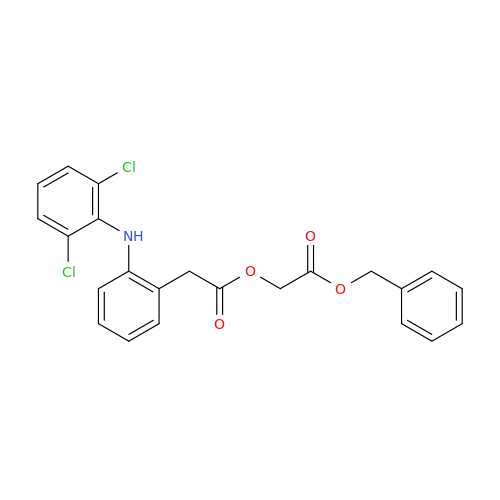
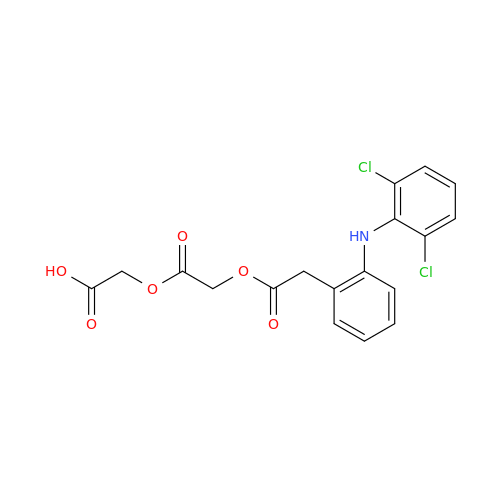
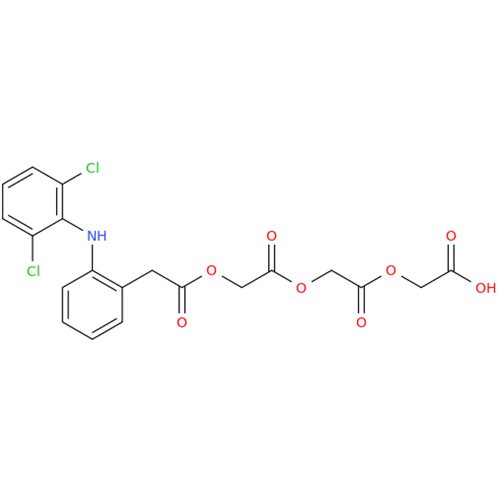
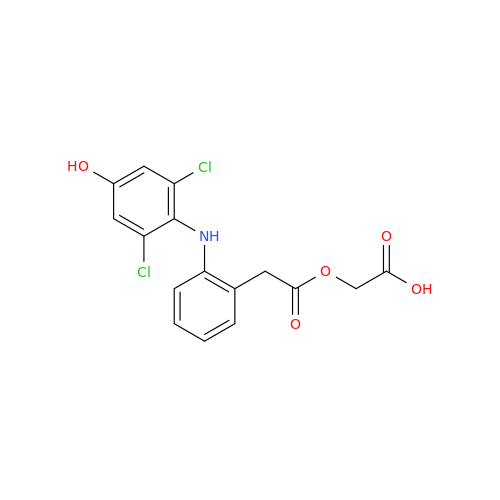
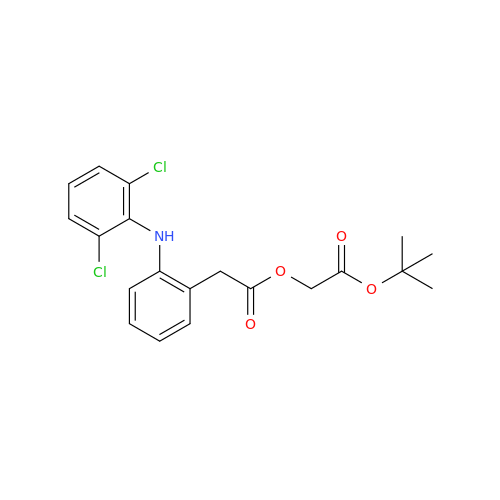
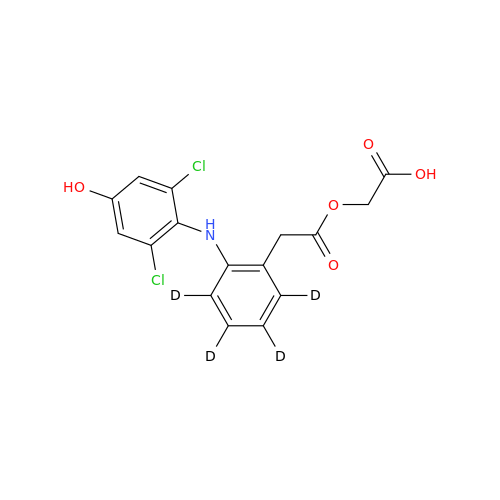
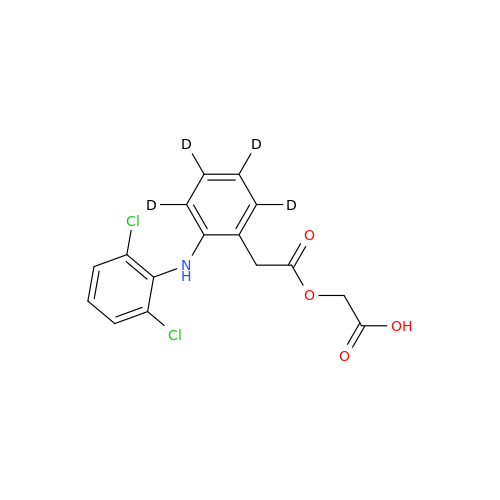
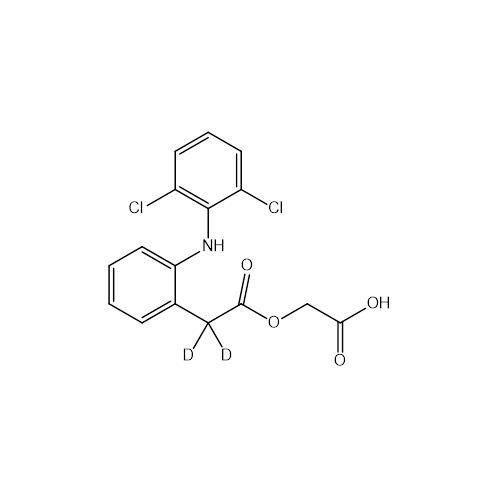
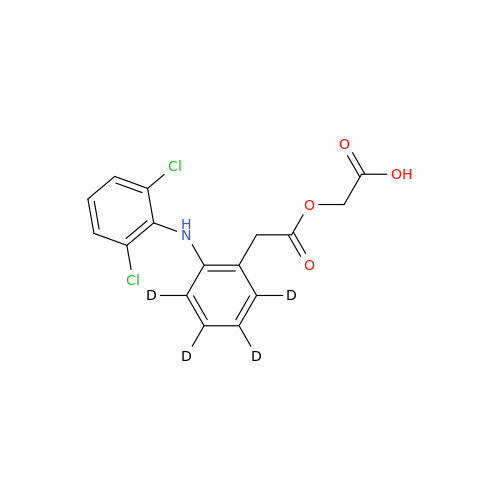
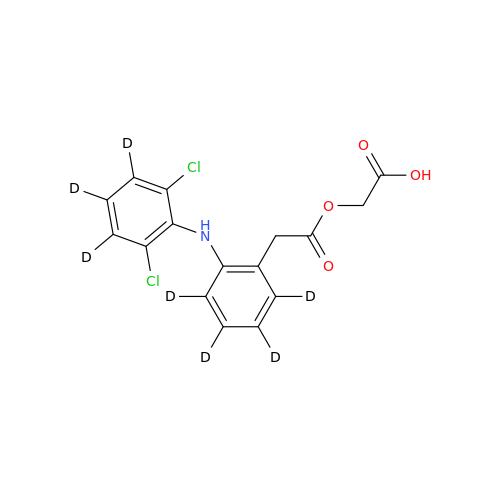
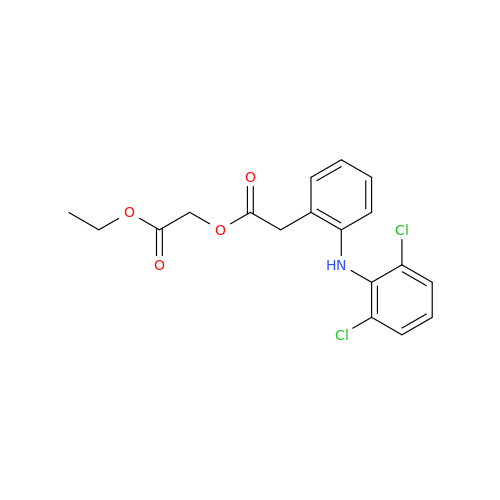
Aceclofenac EP Impurity E
|
Chemical Name: Aceclofenac EP Impurity E
Synonym: Ethyl [[[2-[(2,6-dichlorophenyl)amino]phenyl]acetyl]oxy]acetate; 2-Ethoxy-2-oxoethyl 2-(2-((2,6-dichlorophenyl)amino)phenyl)acetate; 2-[(2,6-Dichlorophenyl)amino]benzeneacetic Acid 2-Ethoxy-2-oxoethyl Ester| Enter Batch Number | |||