Product Information
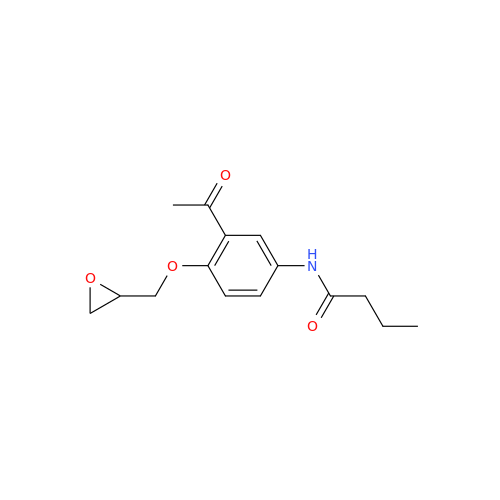
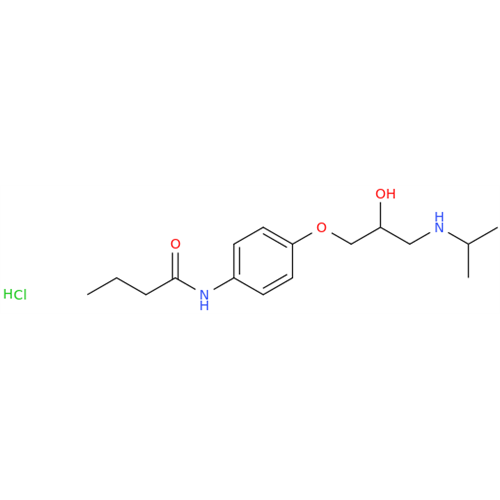
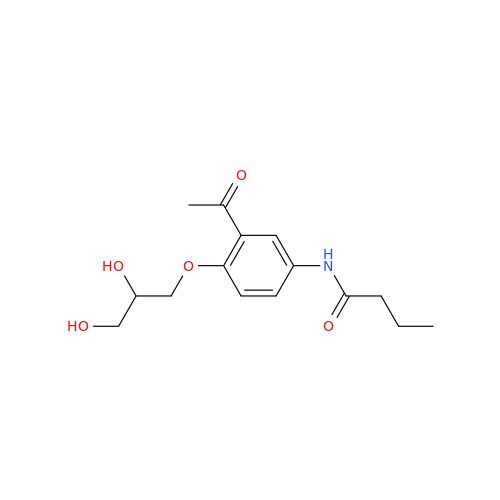
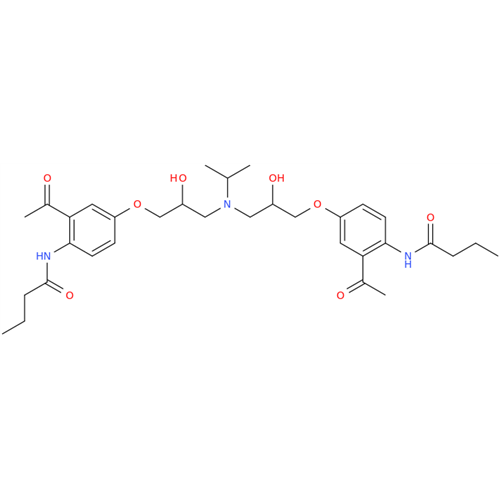
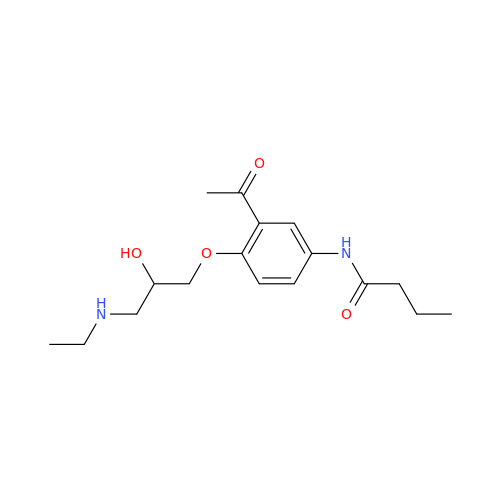
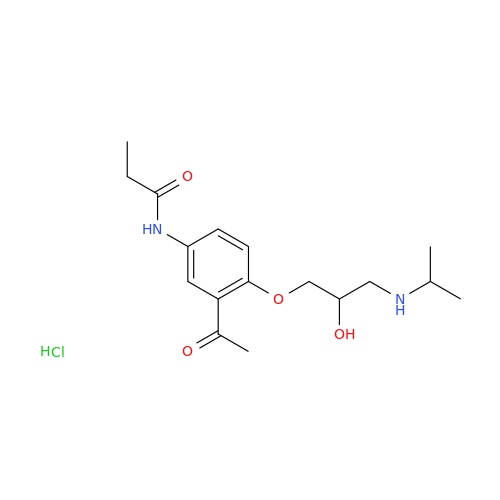
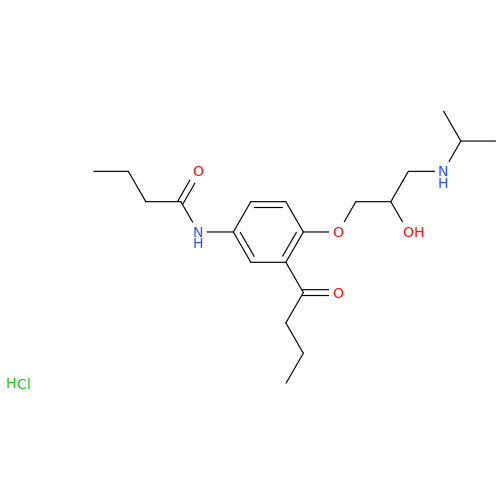
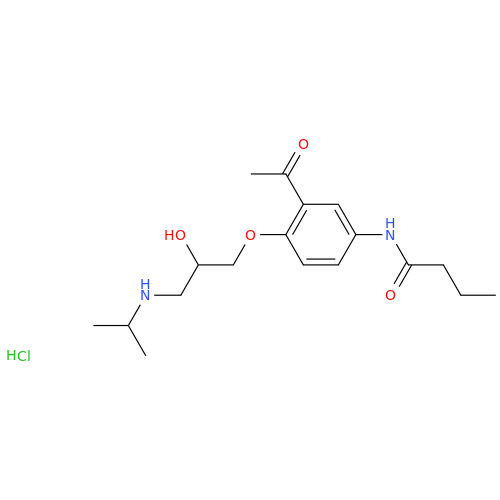
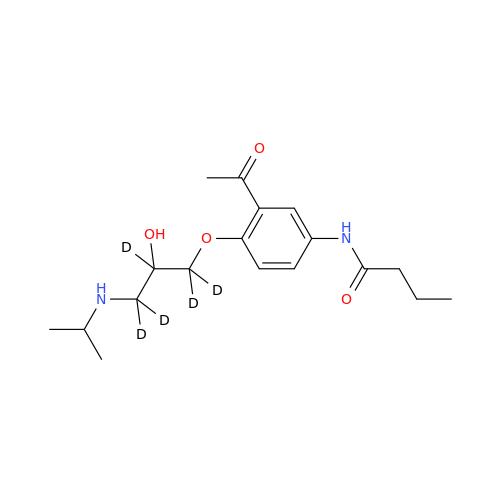
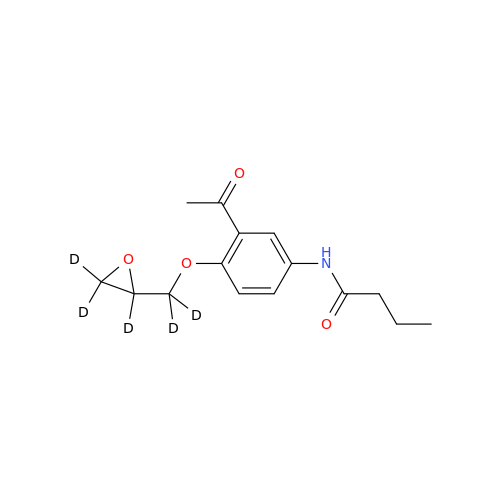
Acebutolol EP Impurity H
|
Chemical Name: Acebutolol EP Impurity H
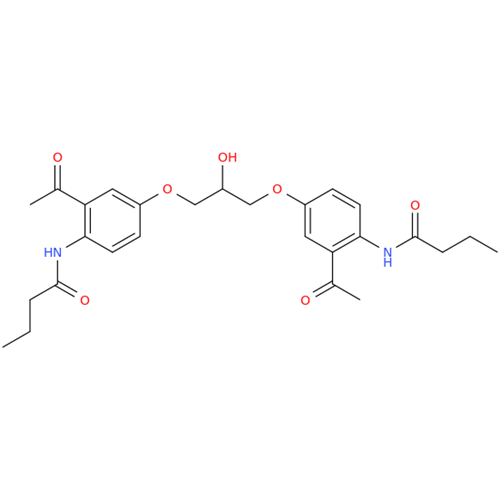
Synonym: [(2-Hydroxypropane-1,3-diyl)bis[oxy(3-acetyl-1,4-phenylene)]]dibutanamide, N,N'-; N,N'-[(2-Hydroxypropane-1,3-diyl)bis[oxy(3-acetyl-1,4-phenylene)]]dibutanamide| Enter Batch Number | |||