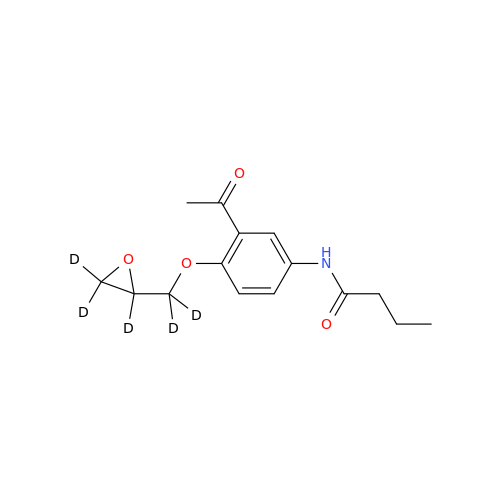
Product Information
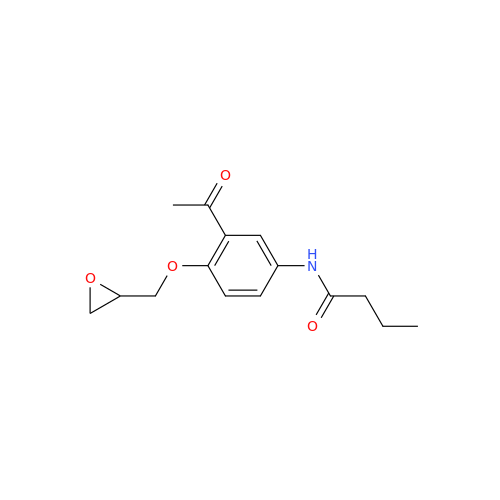
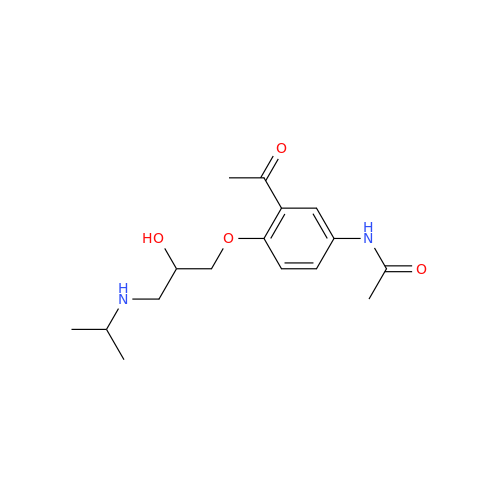
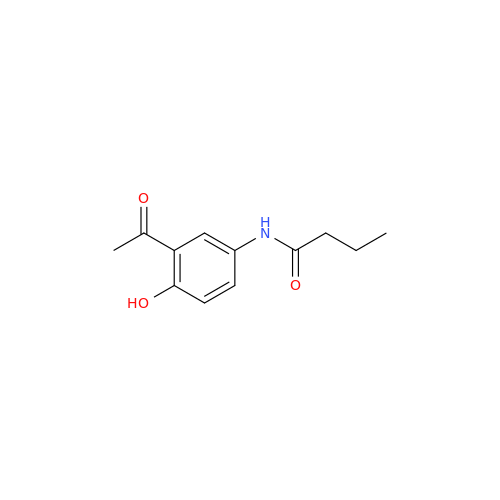
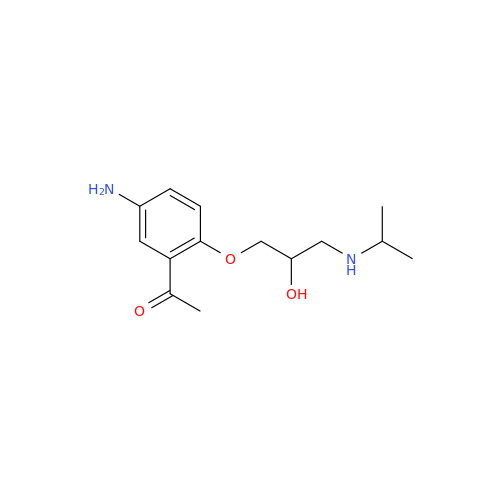
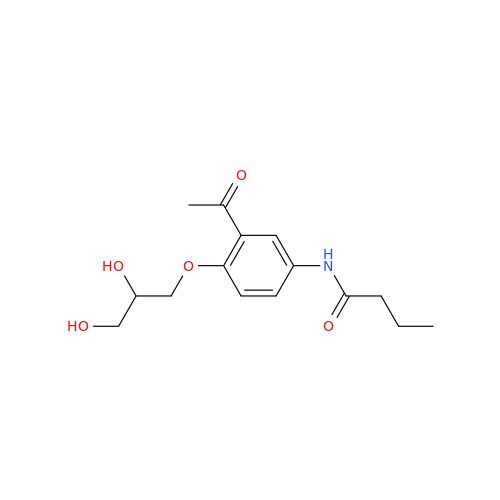
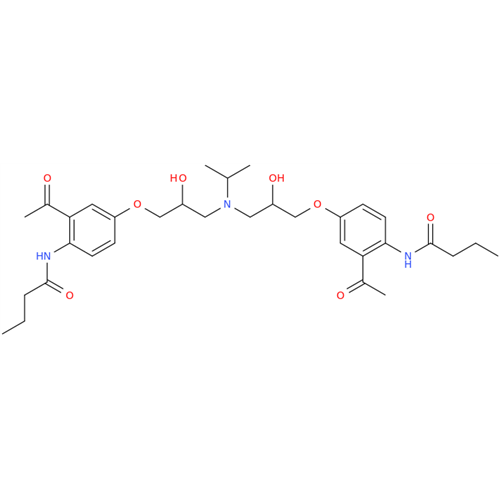
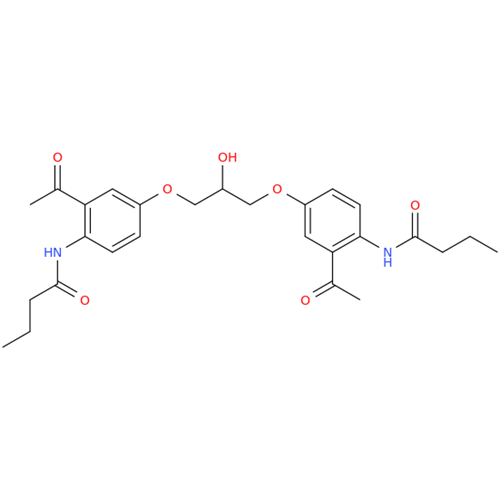
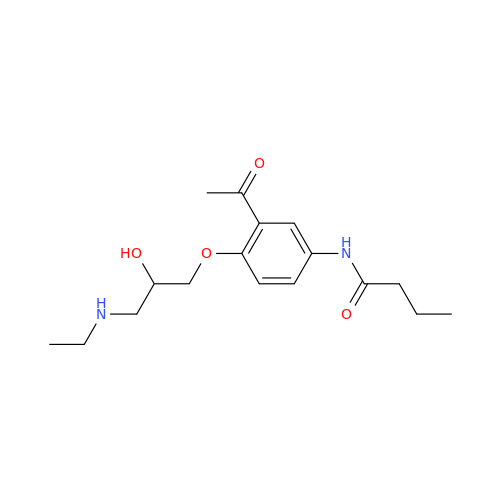
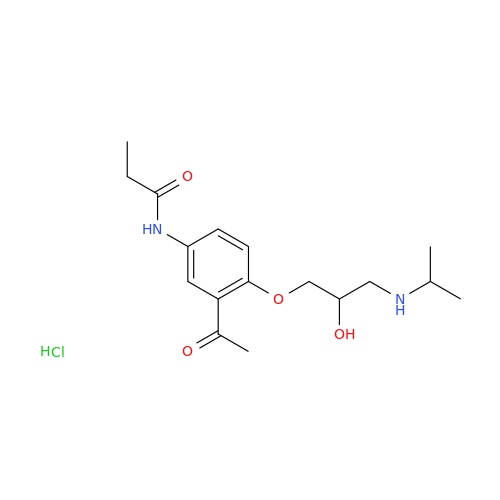
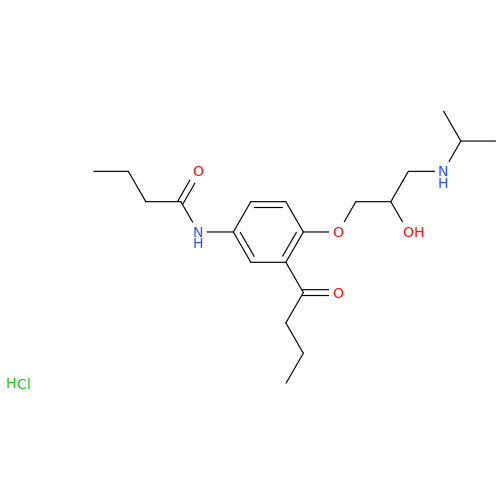
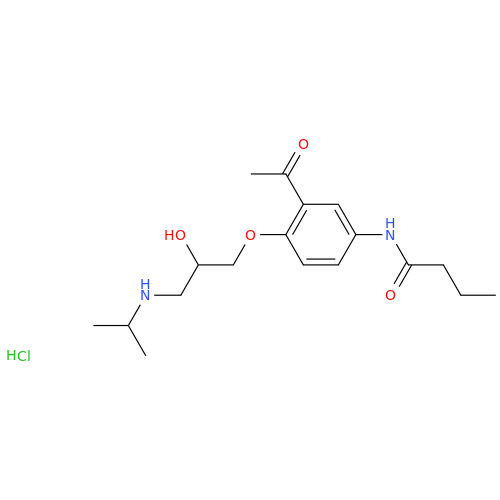
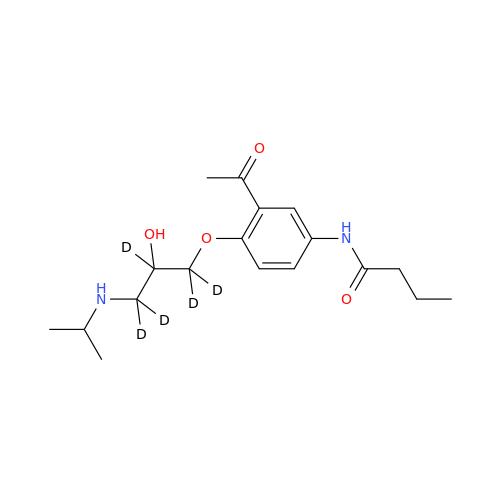
Acebutolol Hydrochloride - Impurity E (Hydrochloride Salt)
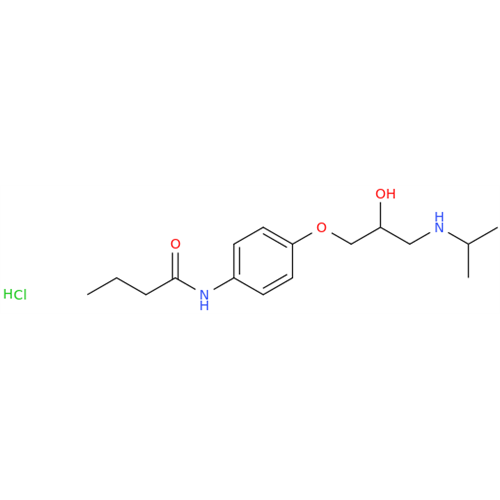
|
Chemical Name: Acebutolol Hydrochloride - Impurity E (Hydrochloride Salt)
Synonym: N-[4-[(2RS)-2-Hydroxy-3-[(1-methylethyl)amino]propoxy]phenyl]butanamide Hydrochloride| Enter Batch Number | |||