Product Information
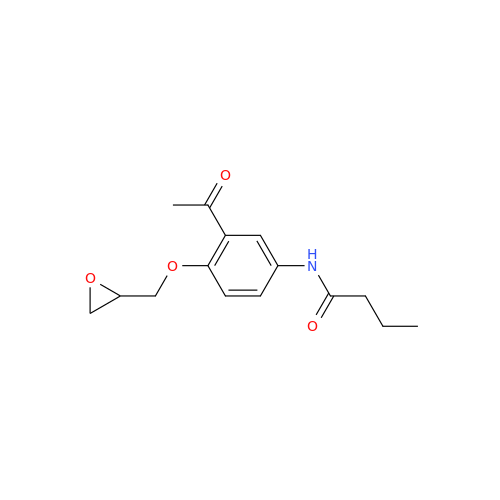
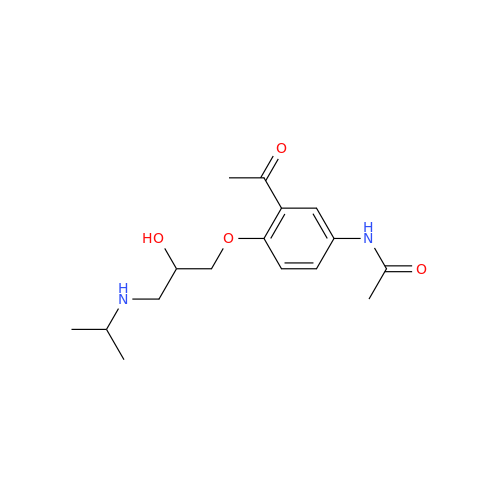
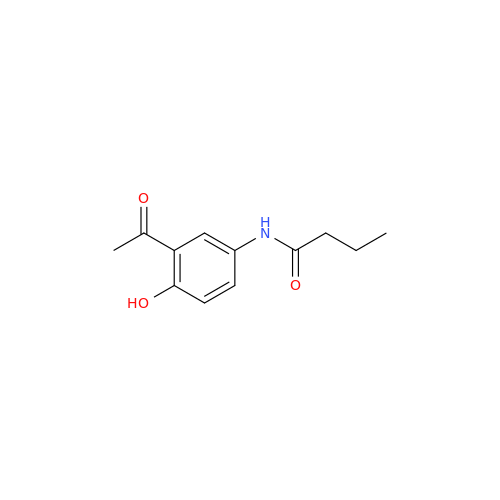
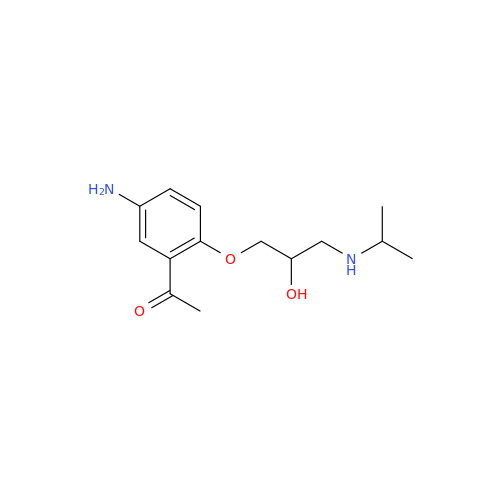
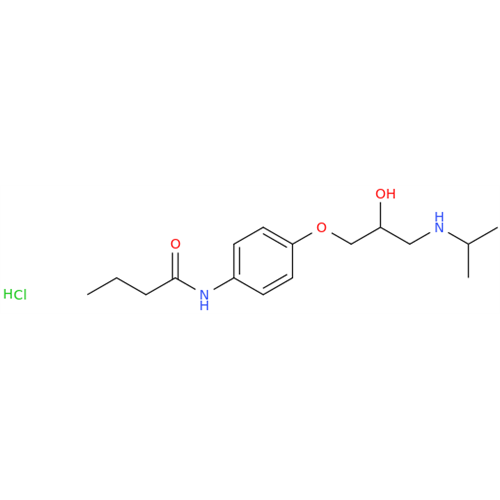
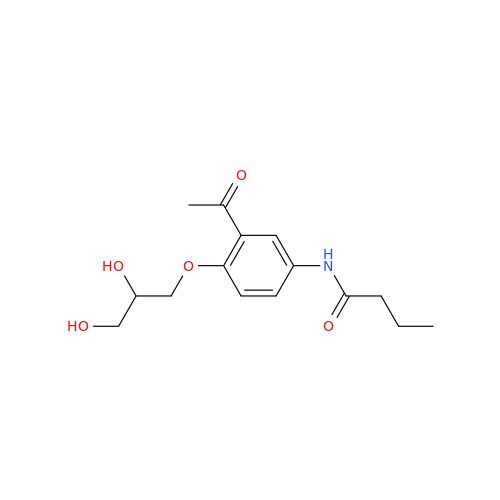
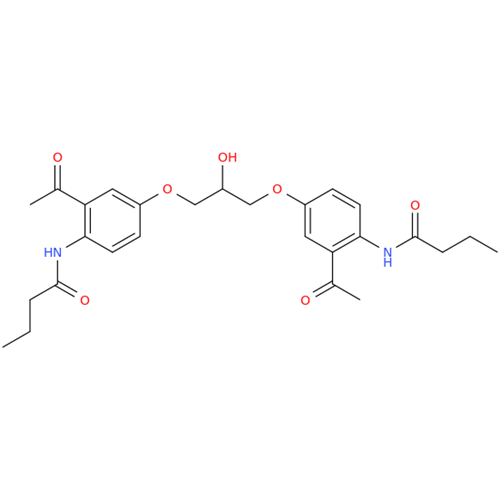
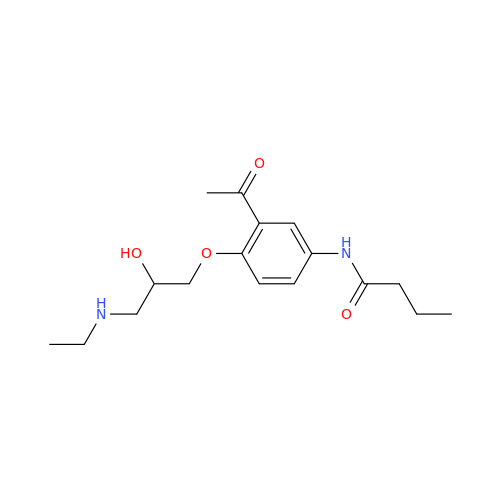
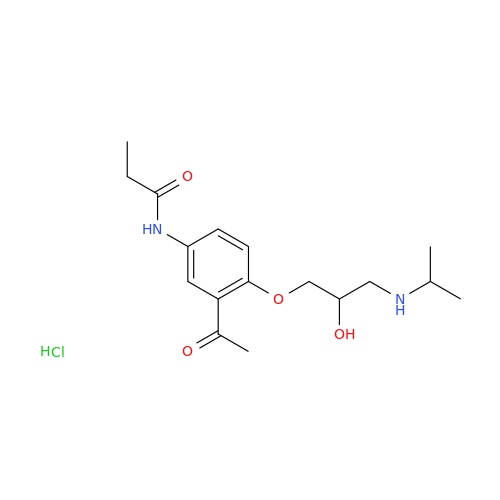
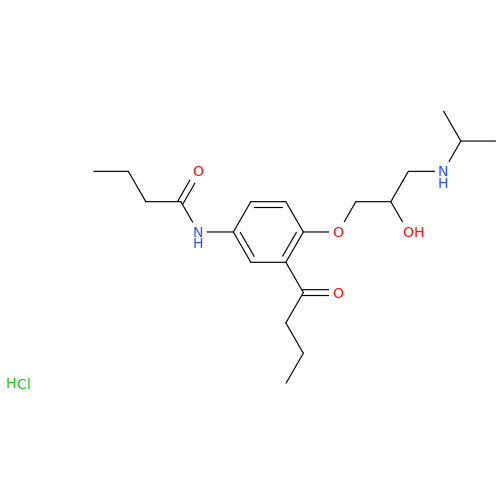
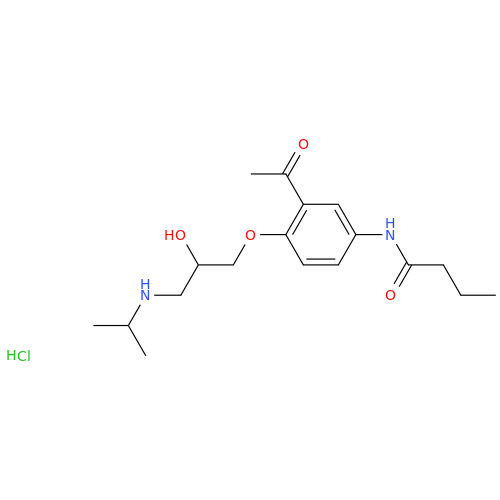
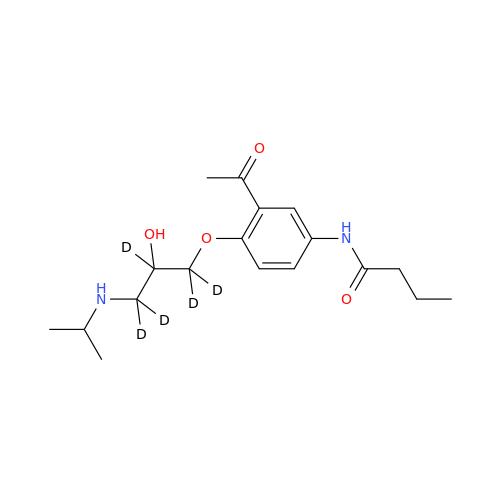
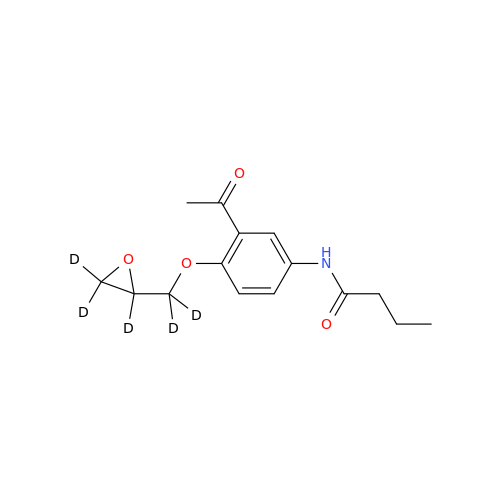
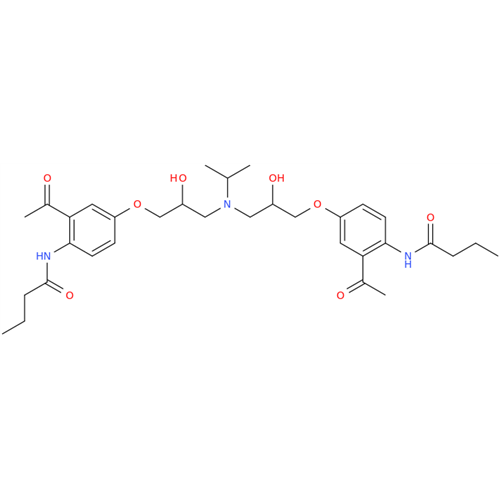
Acebutolol EP Impurity G
|
Chemical Name: Acebutolol EP Impurity G
Synonym: N,N'-[[(1-Methylethyl)imino]-bis[(2-hydroxypropane-1,3-diyl)oxy(3-acetyl-1,4-phenylene)]]dibutanamide; N,N'-((((isopropylazanediyl)bis(2-hydroxypropane-3,1-diyl))bis(oxy))bis(2-acetyl-4,1-phenylene))dibutyramide; Acebutolol Dimer Impurity| Enter Batch Number | |||