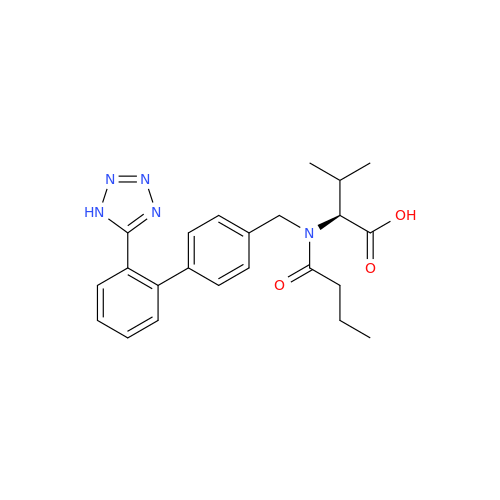
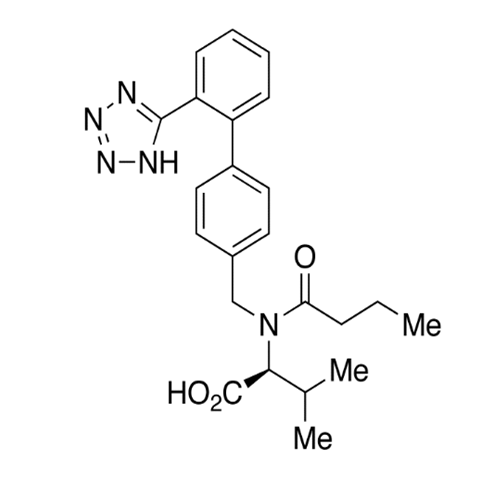
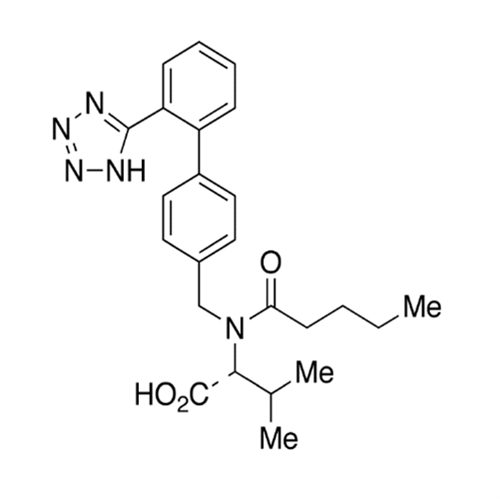
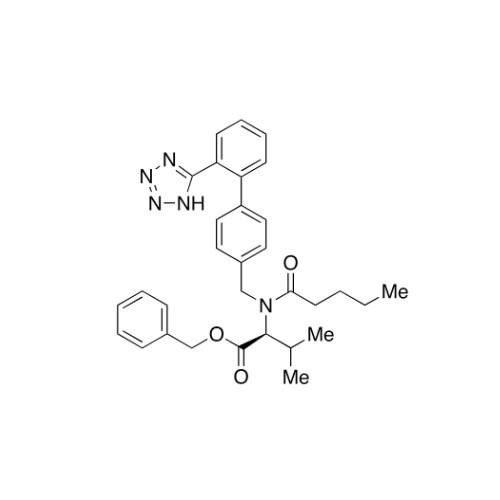
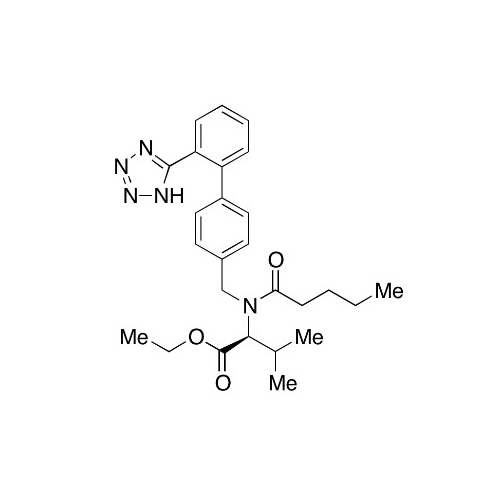
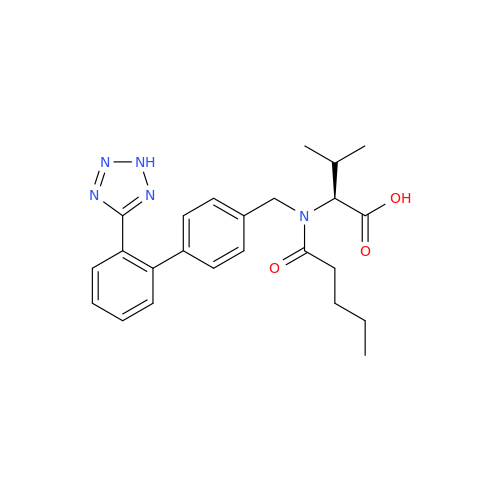
Chemical Name: Valsartan EP Impurity C
Synonym: N-((2'-(1H-tetrazol-5-yl)-[1,1'-biphenyl]-4-yl)methyl)-N-butyryl-L-valine; Valsartan n-Propyl Impurity; (S)-N-butyryl-N-{[2’-(1H-tetrazole-5-yl)-biphenyl-4-yl]-methyl}-valine; N-(1-Oxobutyl)-N-[[2'-(2H-tetrazol-5-yl)[1,1'-biphenyl]-4-yl]methyl]-L-valine; N-(1-Oxobutyl)-N-[[2'-(2H-tetrazol-5-yl)[1,1'-biphenyl]-4-yl]methyl]-L-valine; N-Butyryl-N-{[2'-(1H-tetrazole-5-yl)-biphenyl-4-yl]-methyl}-L-valine; (2S)-2-[butanoyl[[2'-(1H-tetrazol-5-yl)biphenyl-4-yl]methyl]amino]-3-methylbutanoic acid; N-Butyryl-N-{[2'-(1H-tetrazole-5-yl)biphenyl-4-yl]methyl}-L-valine; Valsartan Impurity B (IP); N-Butyryl-N-{[2'-(1H-tetrazole-5-yl)biphenyl-4-yl]methyl}-L-valine; Valsartan Related Compound B (USP)