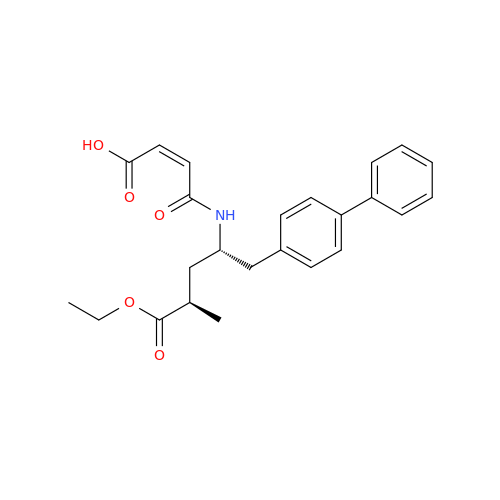
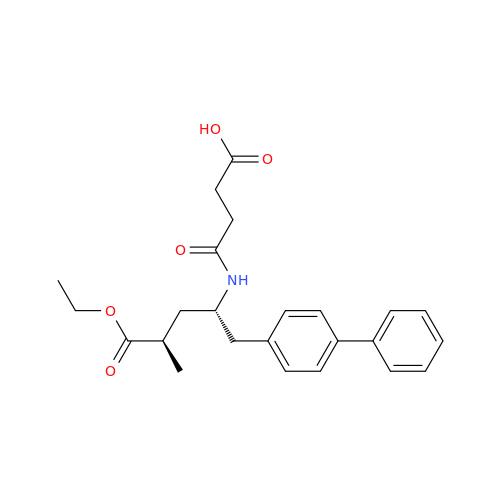
Product Information
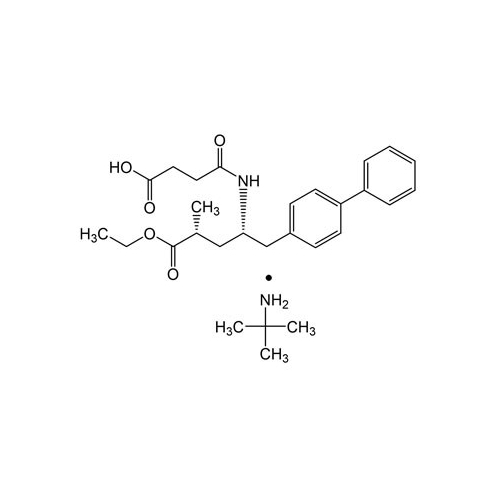
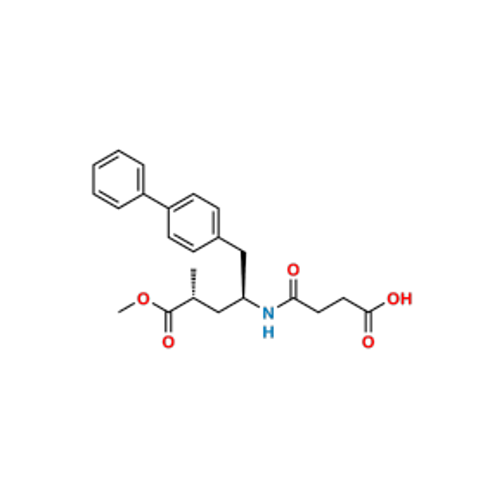
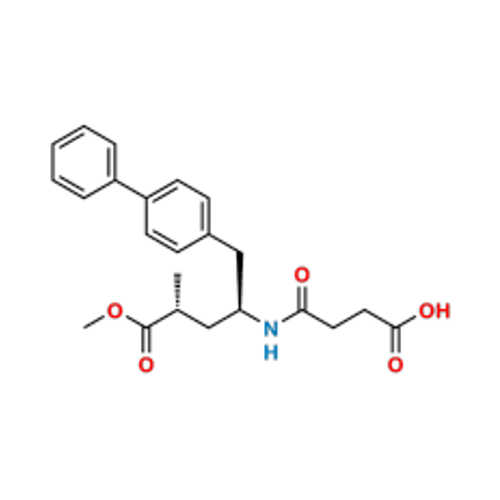
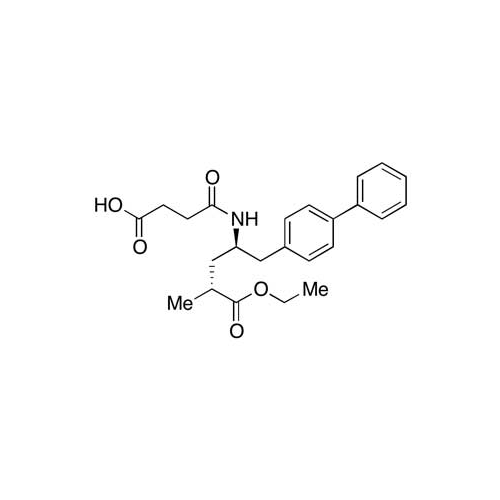
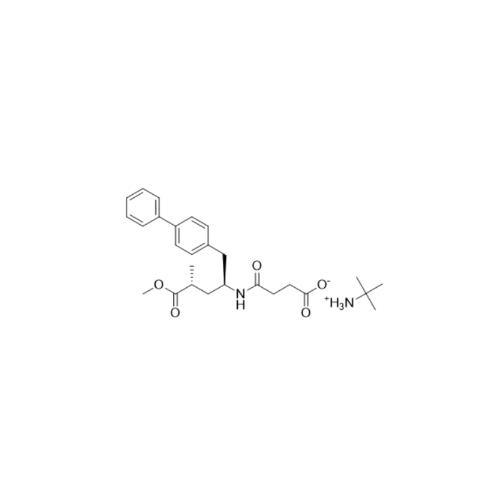
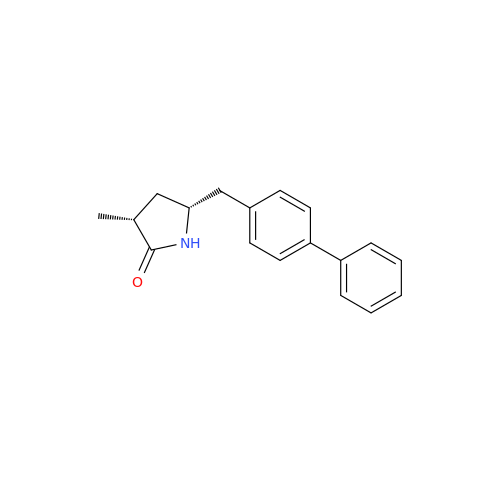
Lactum Impurity of Sacubitril
|
Chemical Name: Lactum Impurity of Sacubitril
Synonym: rel-(3R,5R)-5-([1,1′-Biphenyl]-4-ylmethyl)-3-methyl-2-pyrrolidinone| Enter Batch Number | |||