Product Information
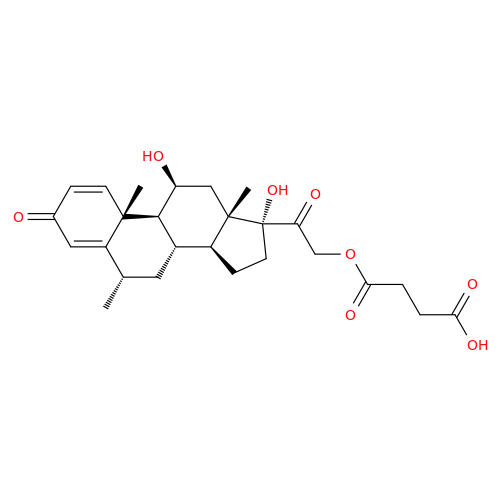
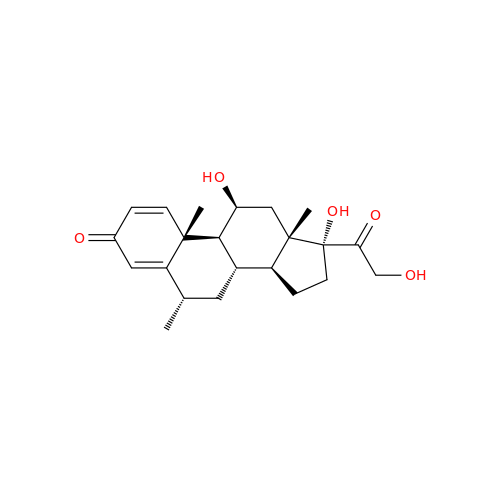
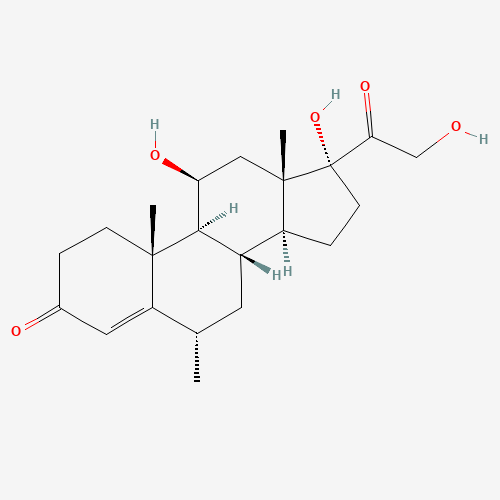
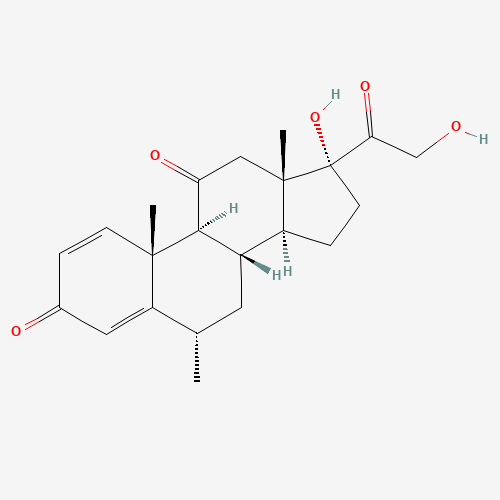
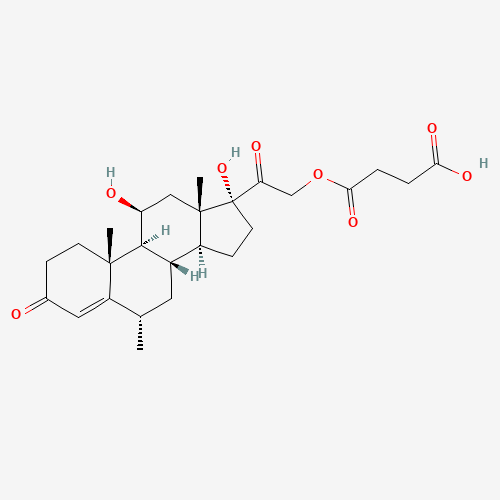
Methylprednisolone Hydrogen Succinate EP Impurity D
|
Chemical Name: Methylprednisolone Hydrogen Succinate EP Impurity D
Synonym: Methylprednisolone Hemisuccinate USP Related Compound D ; methylhydrocortisone 21-(hydrogen succinate)| Enter Batch Number | |||