Product Information
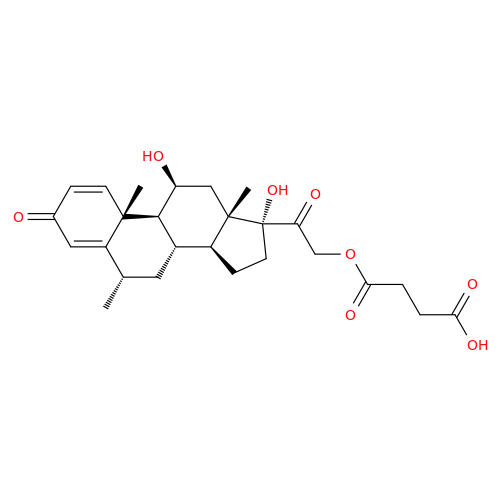
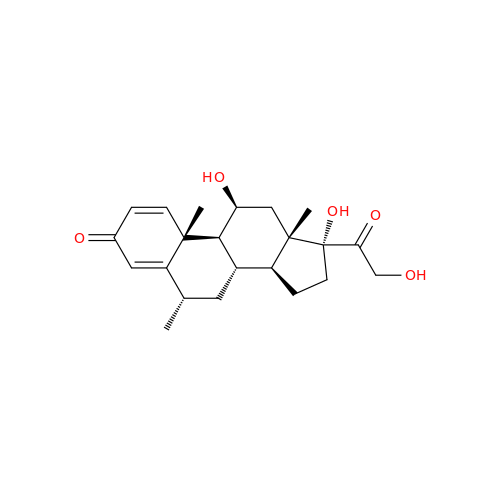
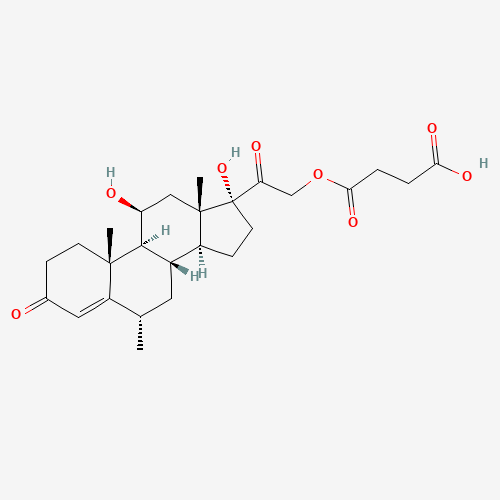
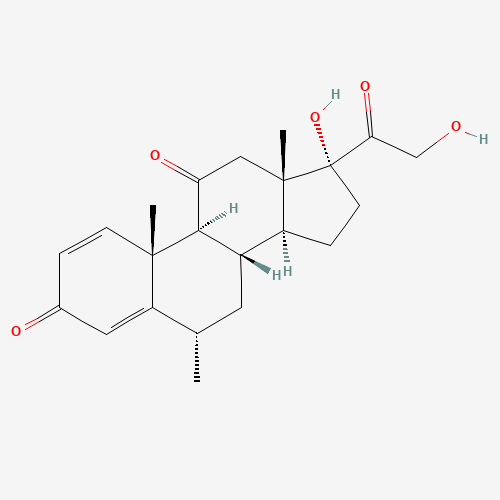
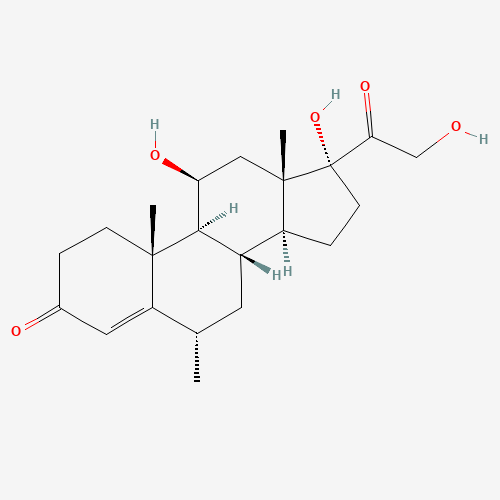
Methylprednisolone EP Impurity F
|
Chemical Name: Methylprednisolone EP Impurity F
Synonym: 6-Alpha Methyl Hydrocortisone ; Methylprednisolone USP related compound F| Enter Batch Number | |||