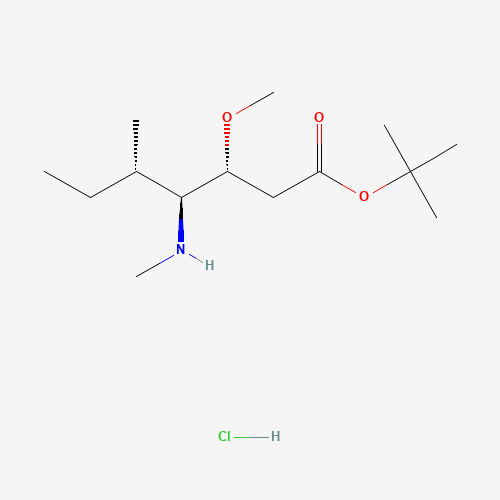
Product Information
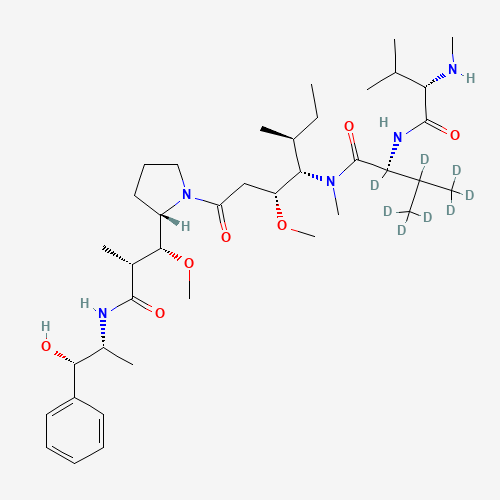
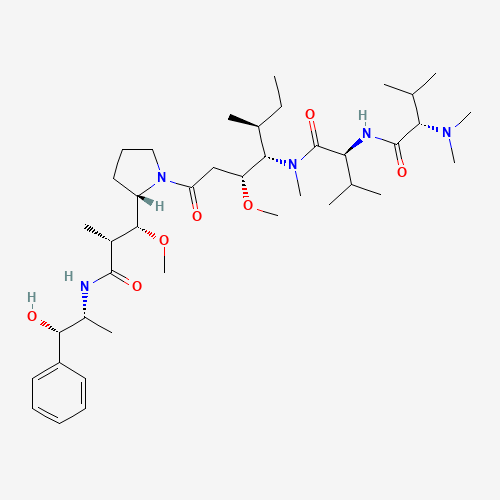
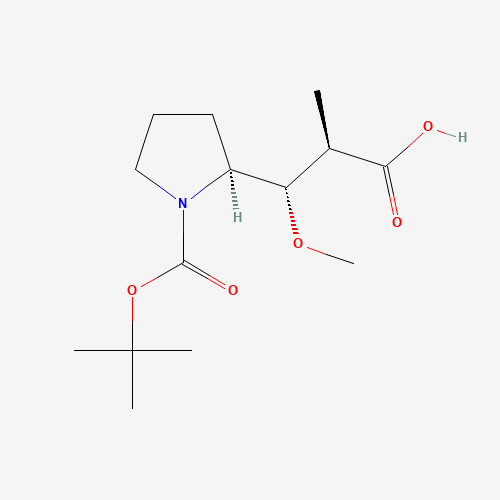
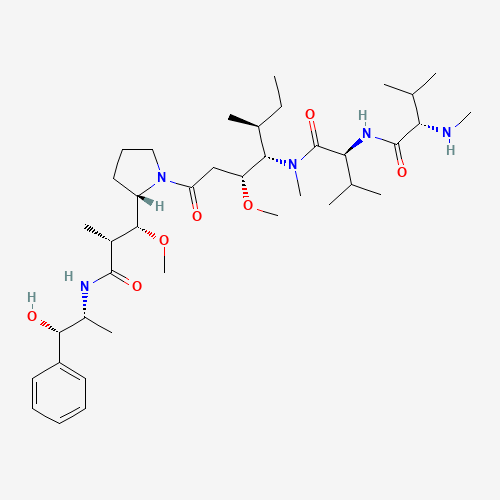
Auristatin E Impurity 1
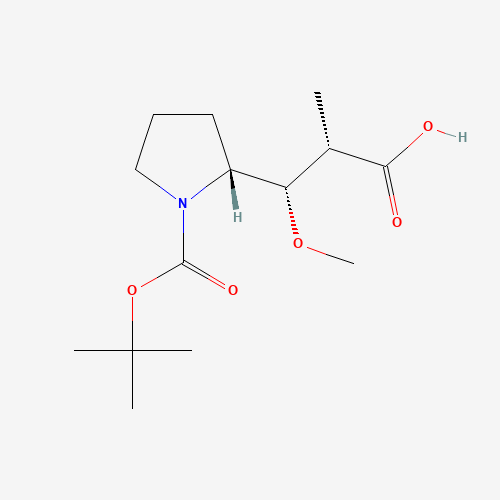
|
Chemical Name: Auristatin E Impurity 1
Synonym: (2S,3S)-3-[(R)-1-Boc-pyrrolidin-2-yl]-3-methoxy-2-methylpropanoic Acid| Enter Batch Number | |||