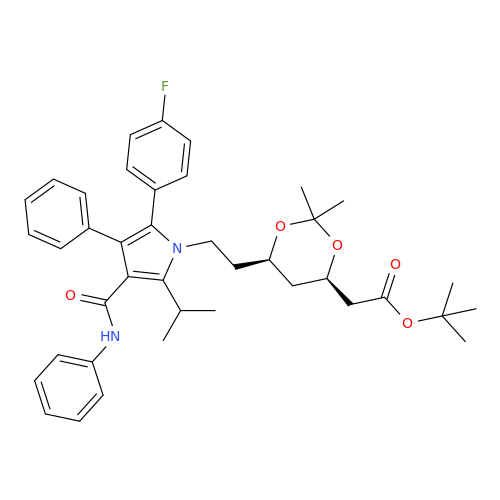
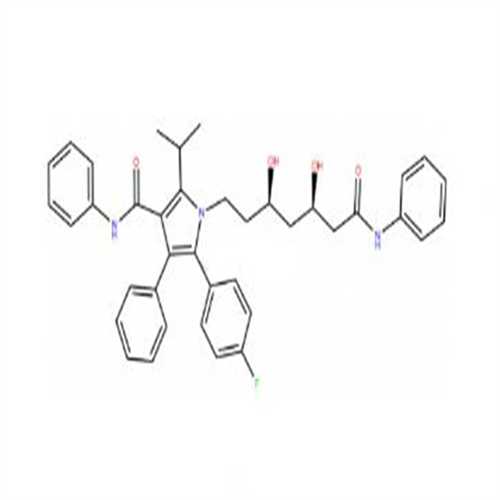
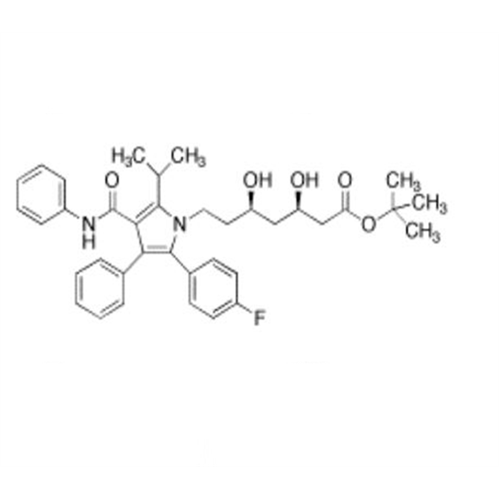
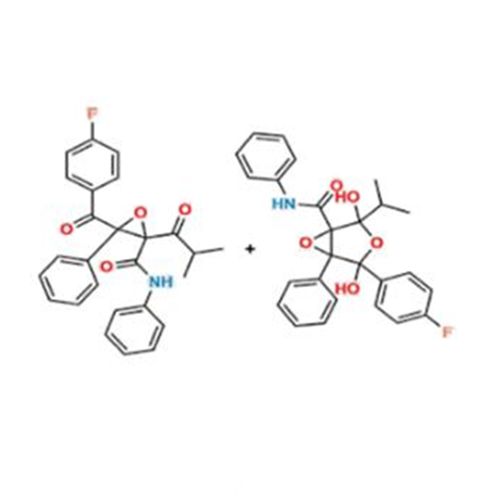
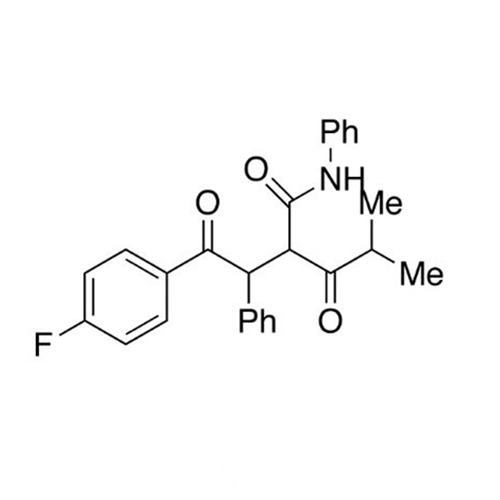
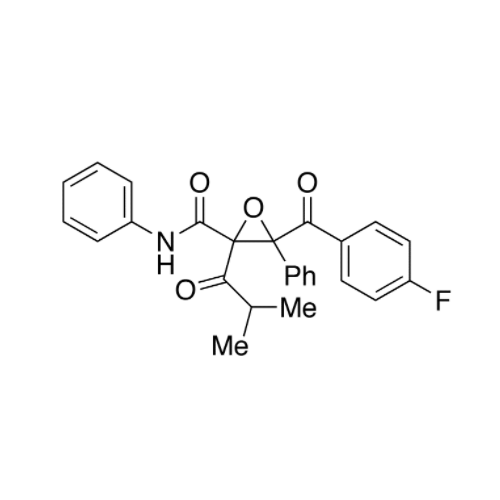
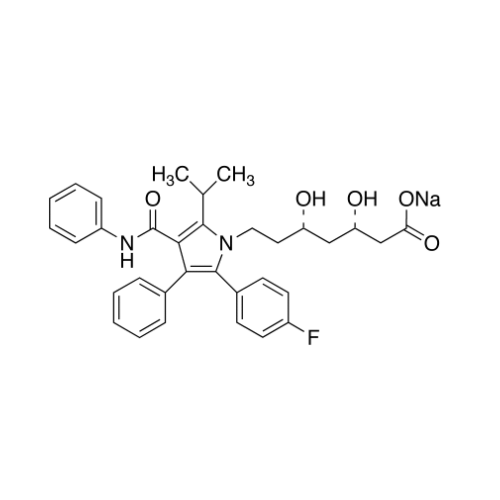
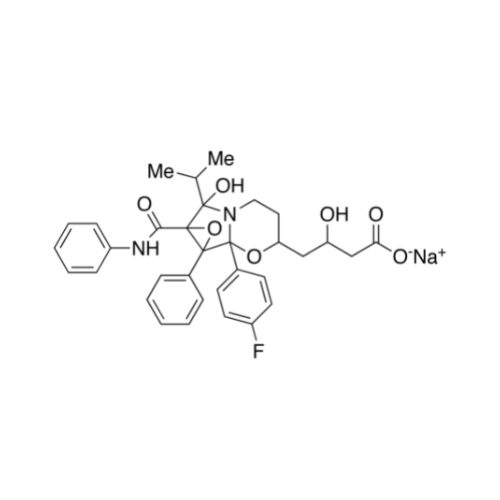
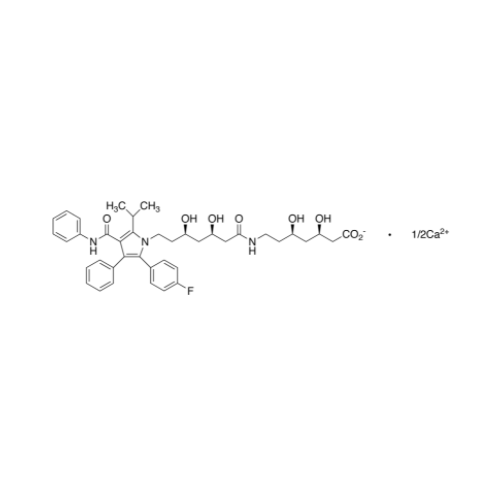
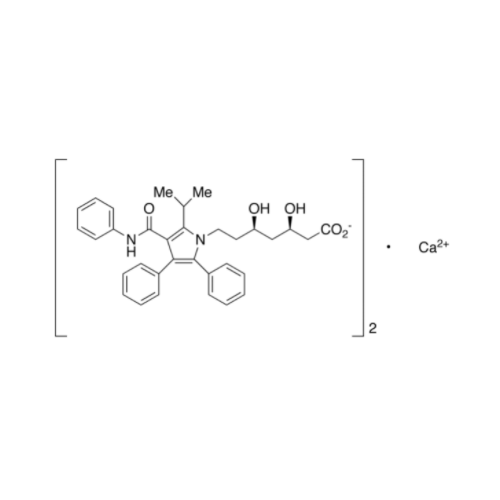
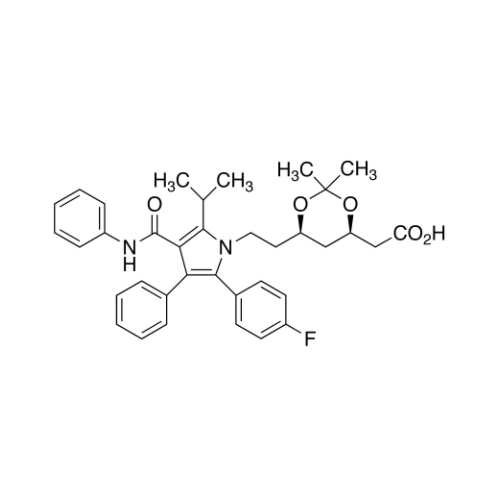
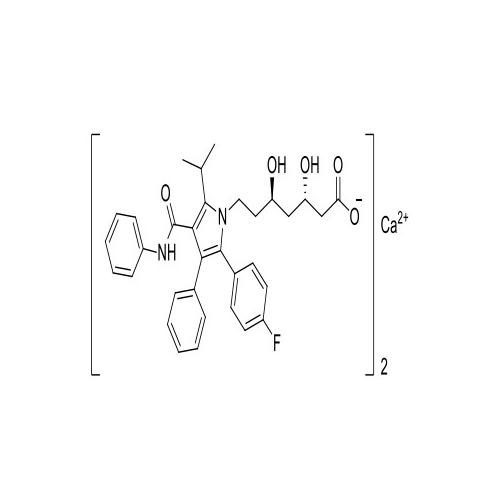
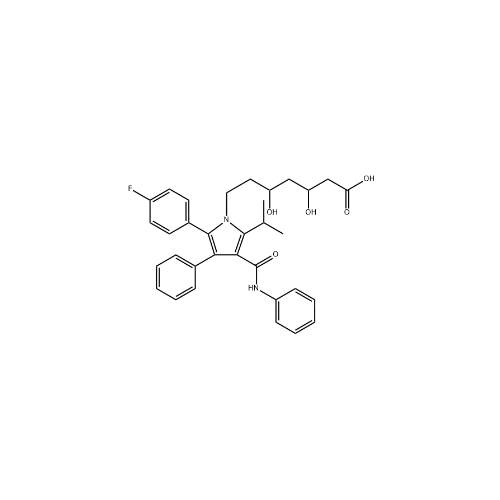
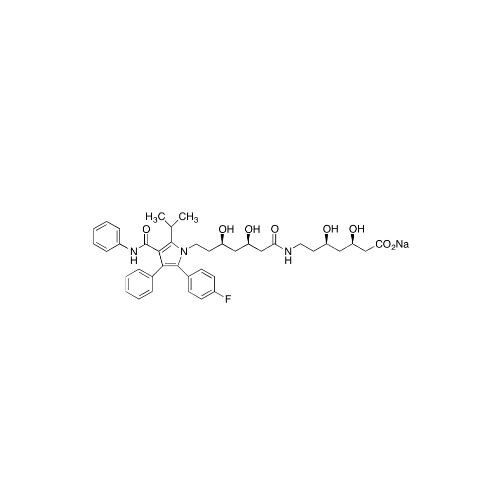
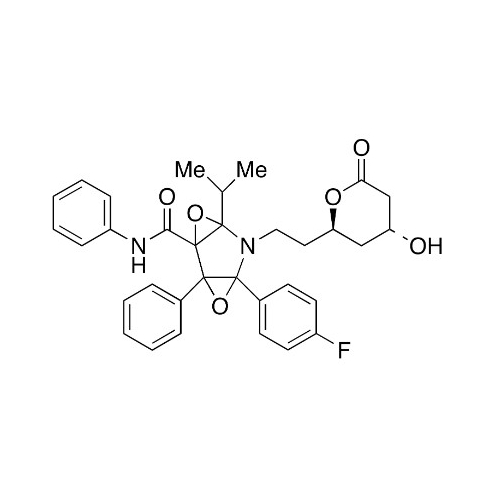
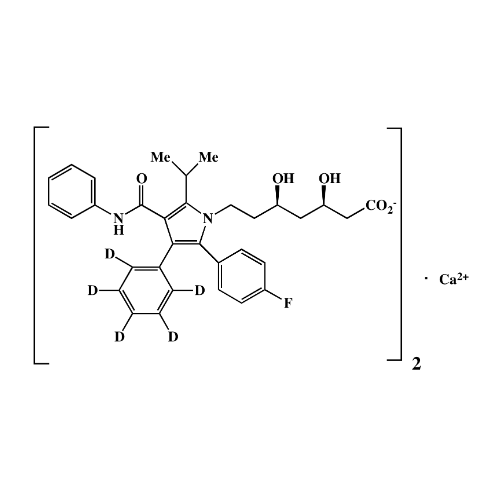
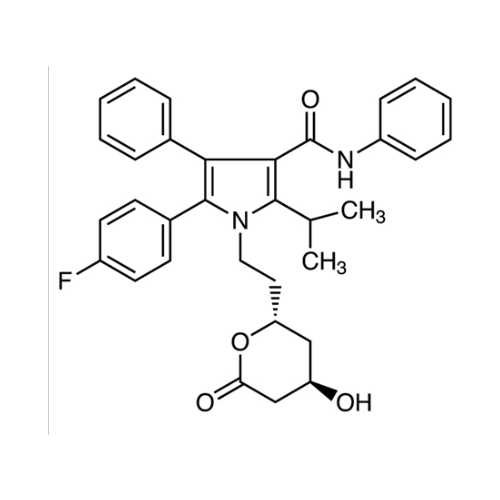
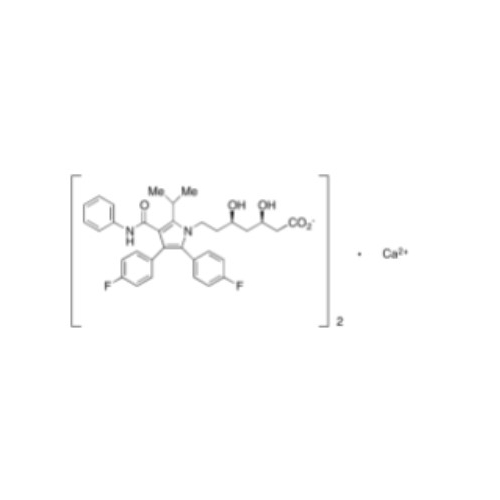
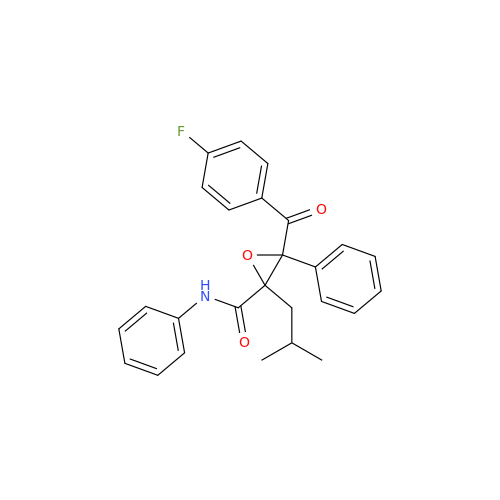
Chemical Name: Atorvastatin EP Impurity I
Synonym: Atorvastatin Calcium Impurity I (As per 35.1 EP Monograph); 1,1-Dimethylethyl 2-[(4R,6R)-6-[2-[2-(4-fluorophenyl)-5-(1-methylethyl)-3-phenyl-4-(phenylcarbamoyl)-1H-pyrrol-1-yl]ethyl]-2,2-dimethyl-1,3-dioxan-4-yl]acetate; Atorvastatin Acetonide tert-Butyl Ester; (4R,6R)-6-[2-[2-(4-Fluorophenyl)-5-(1-methylethyl)-3-phenyl-4-[(phenylamino)carbonyl]-1H-pyrrol-1-yl]ethyl]-2,2-dimethyl-1,3-dioxane-4-acetic Acid 1,1-Dimethylethyl Ester; Atorvastatin Ketal Impurity; Atorvastatin Acetonide impurity; tert-Butyl 2-((4R,6R)-6-{2-[2-(4-fluorophenyl)-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)-1H-pyrrol-1-yl]ethyl}-2,2-dimethyl-1,3-dioxan-4-yl)acetate; Atorvastatin Related Compound I (USP)