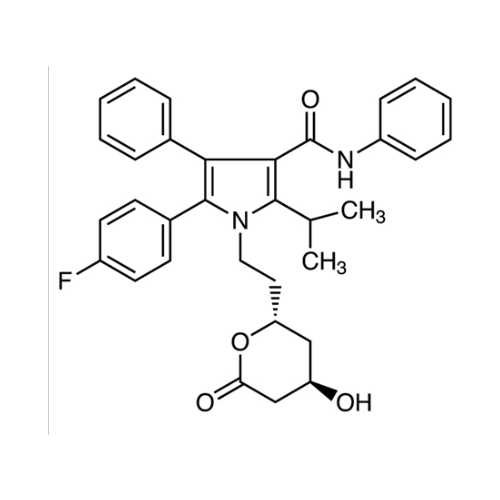
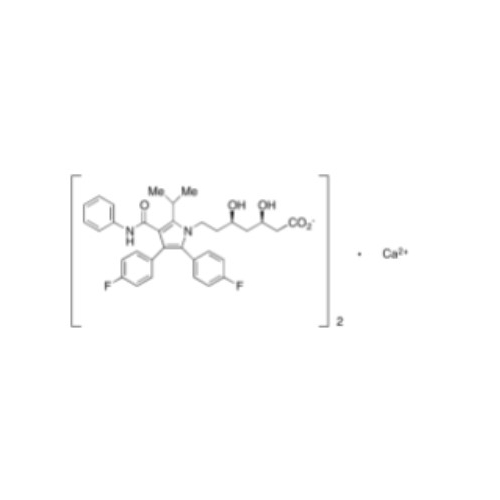
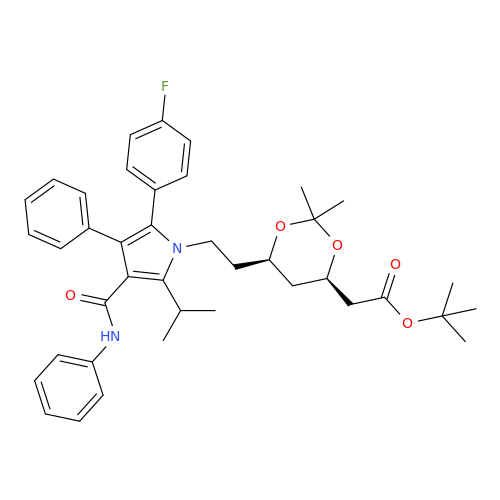
Product Information
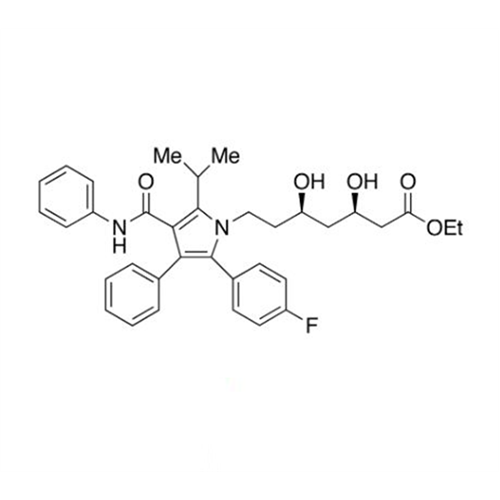
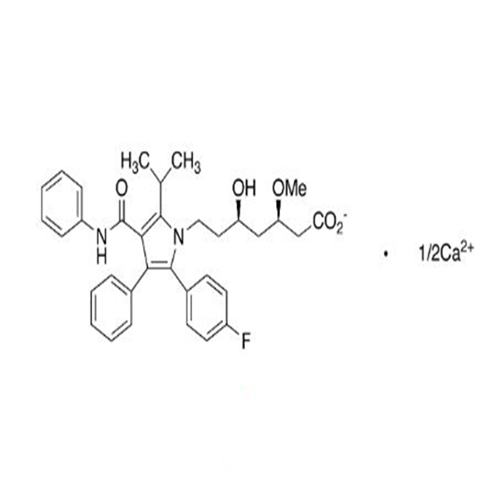
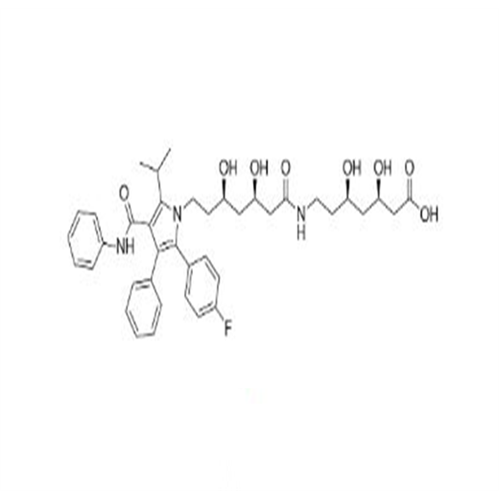
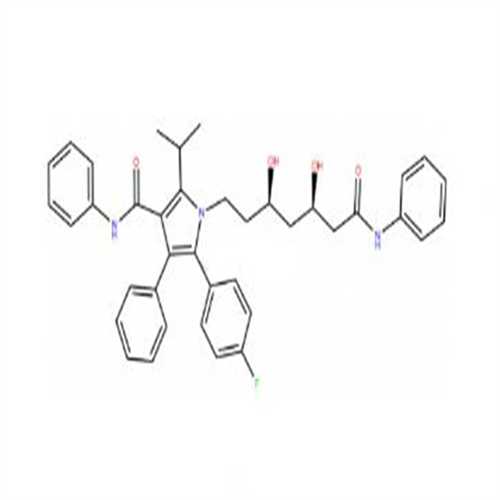
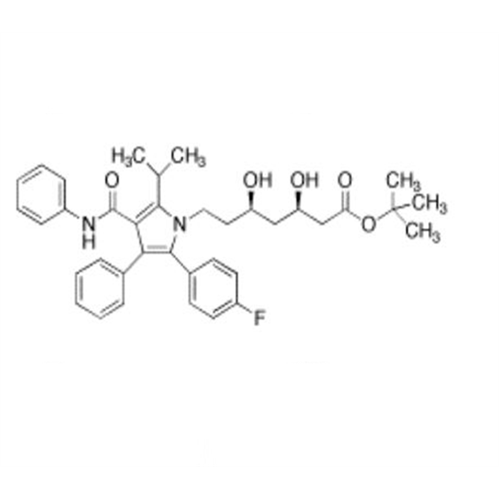
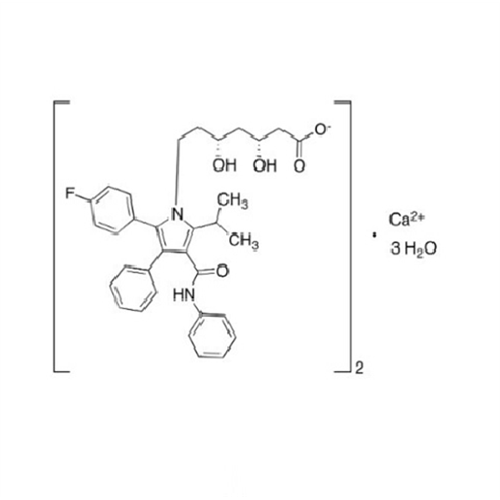
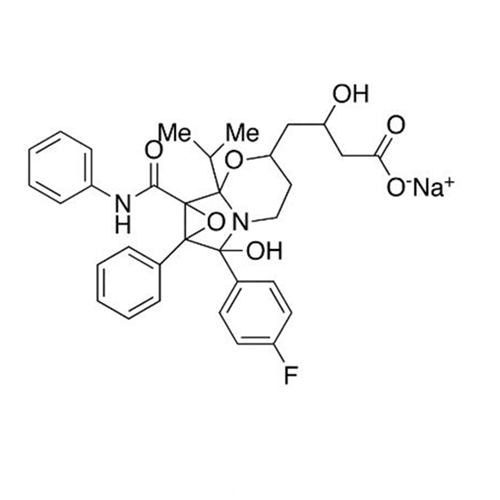
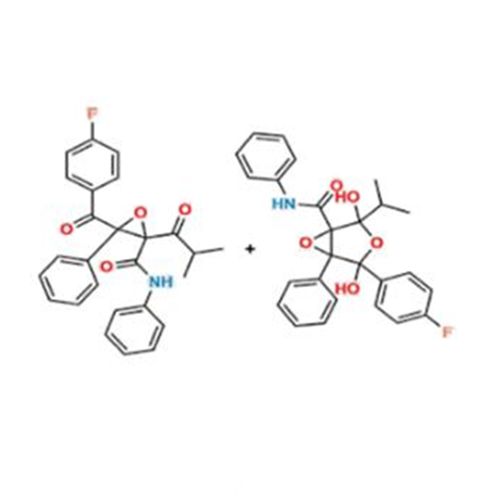
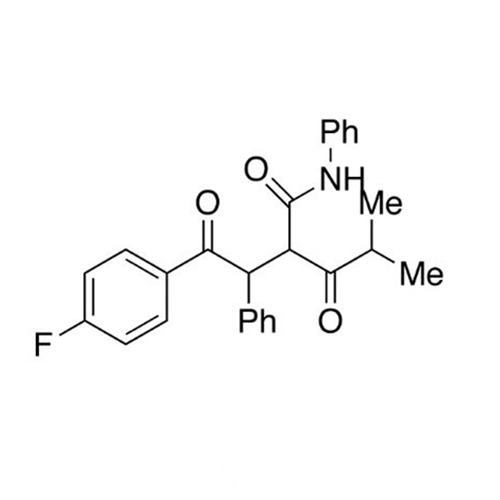
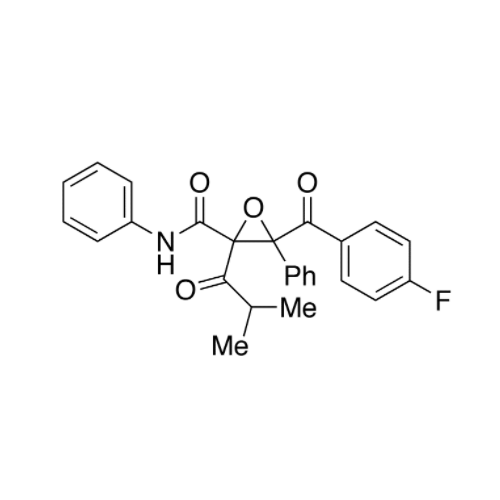
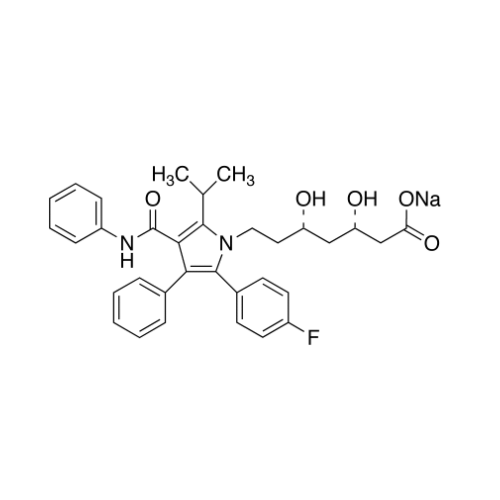
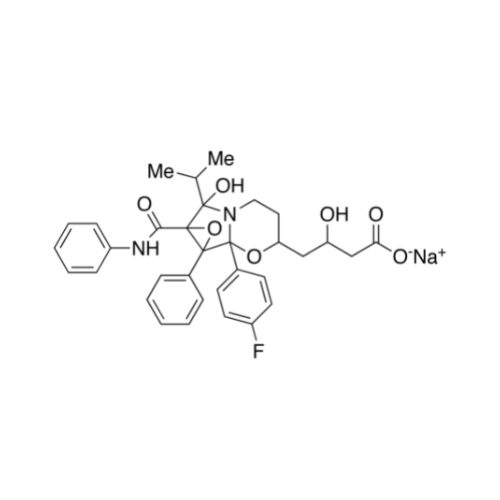
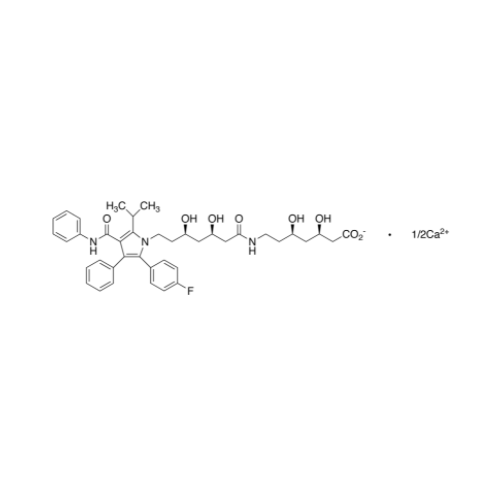
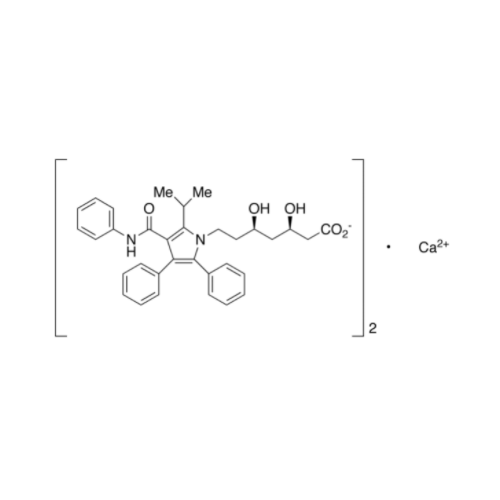
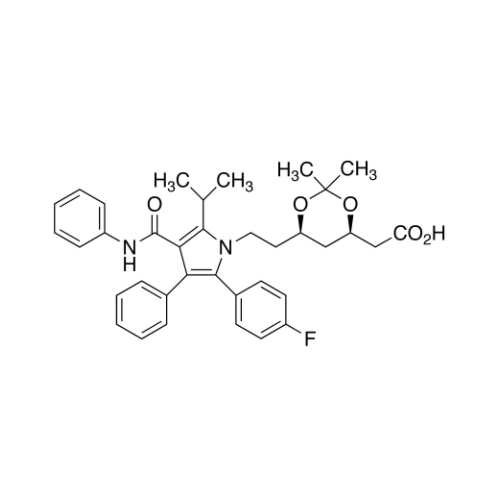
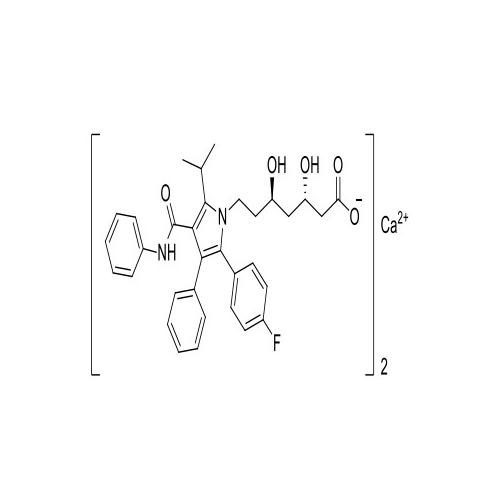
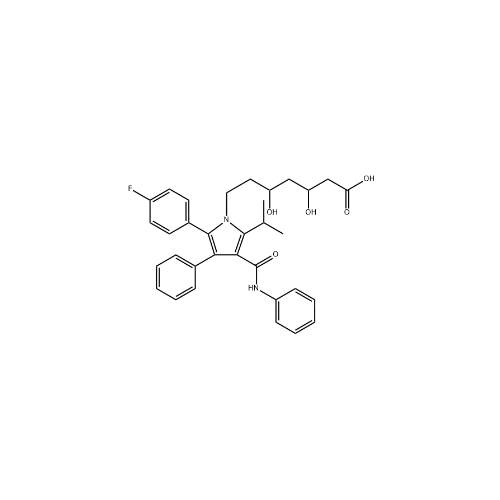
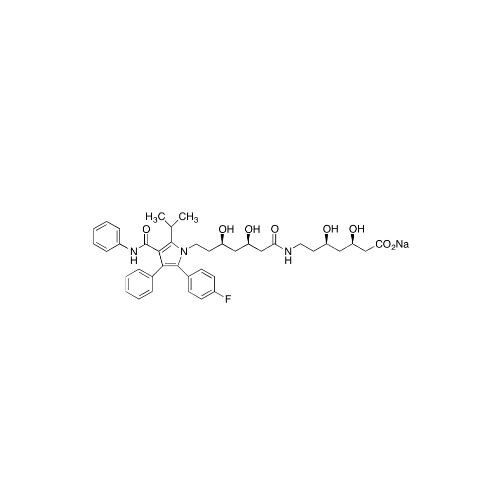
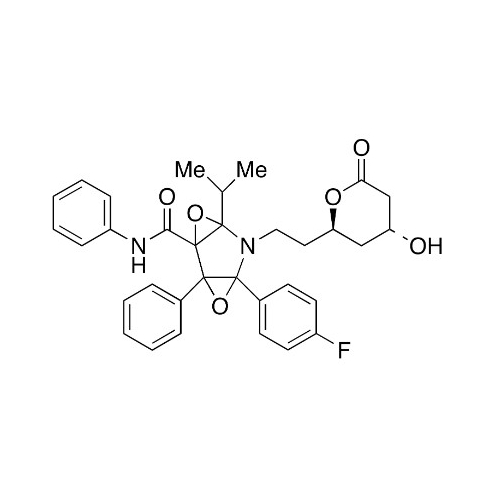
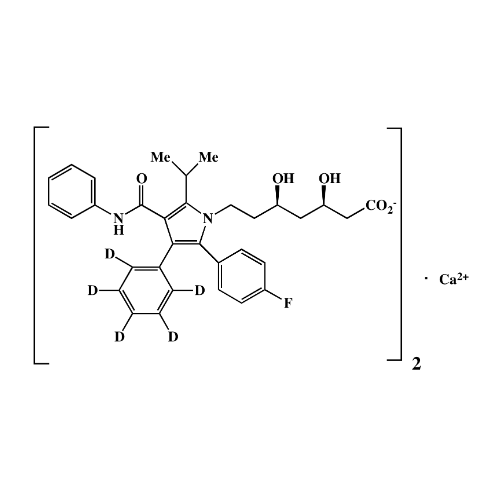
Atorvastatin Oxirane Impurity
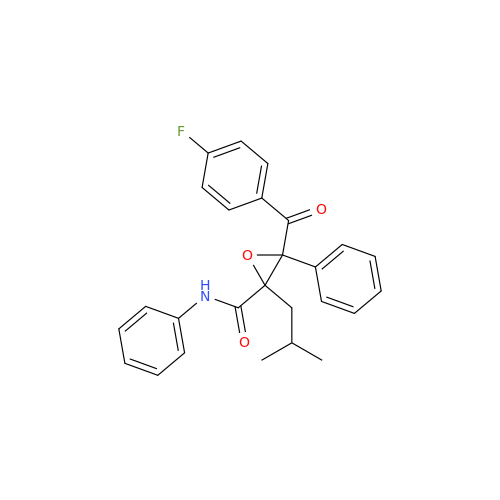
|
Chemical Name: Atorvastatin Oxirane Impurity
Synonym: 3-(4-Fluorobenzoyl)-2-(2-methylpropyl)-N,3-diphenyl-2-oxiranecarboxamide; Atorvastatin Oxirane Impurity (USP)| Enter Batch Number | |||