Product Information
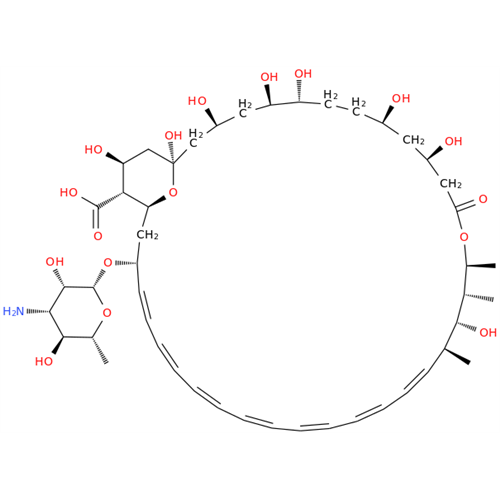
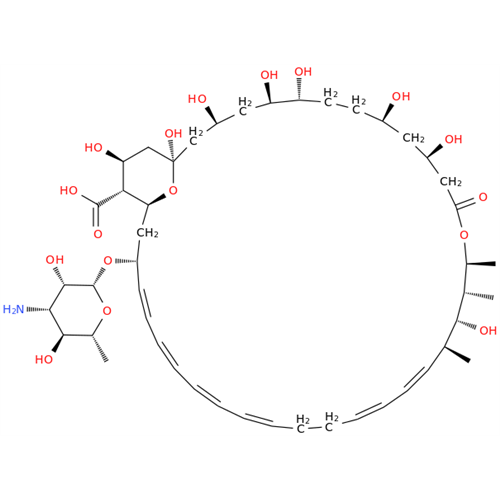
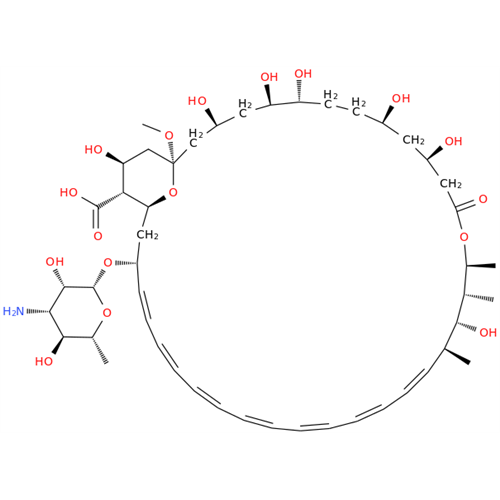
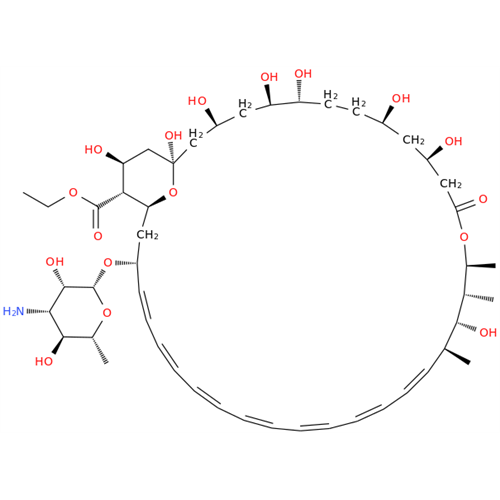
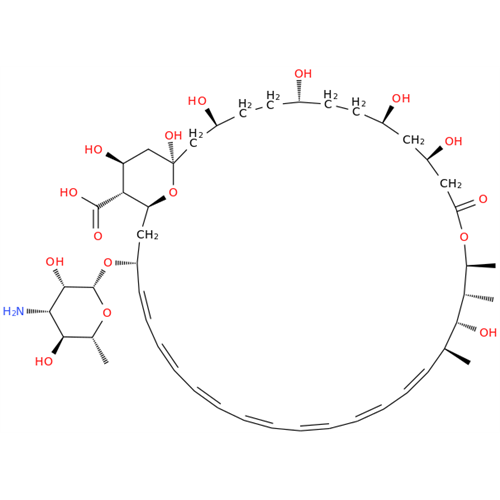
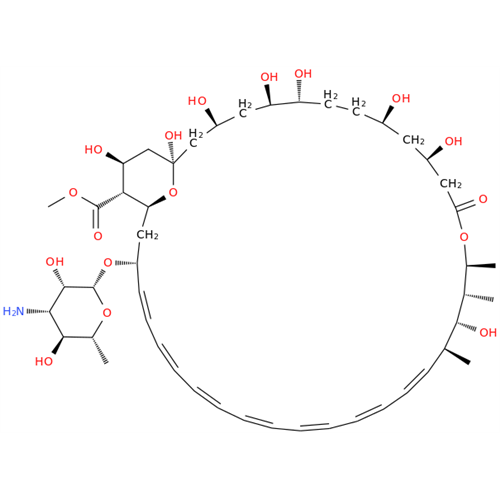
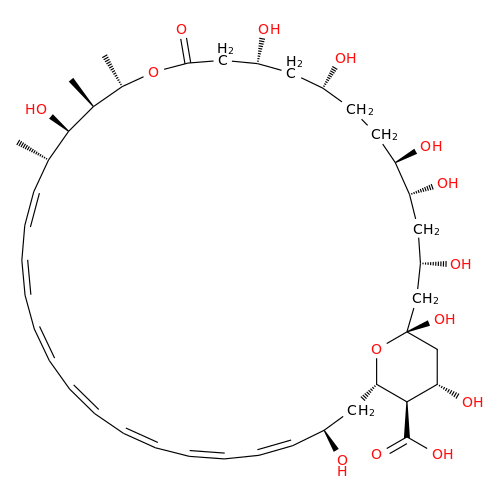
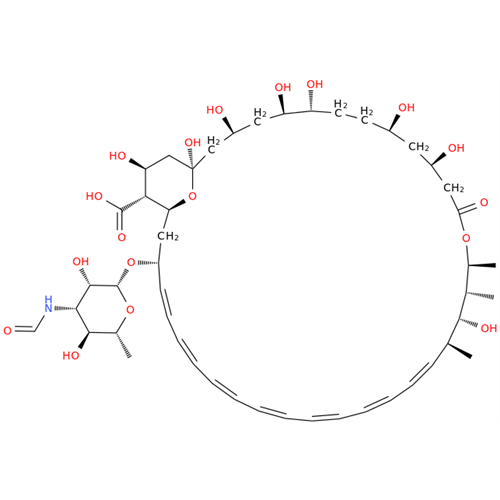
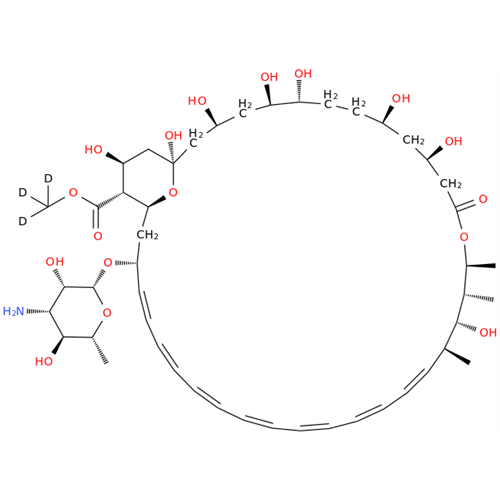
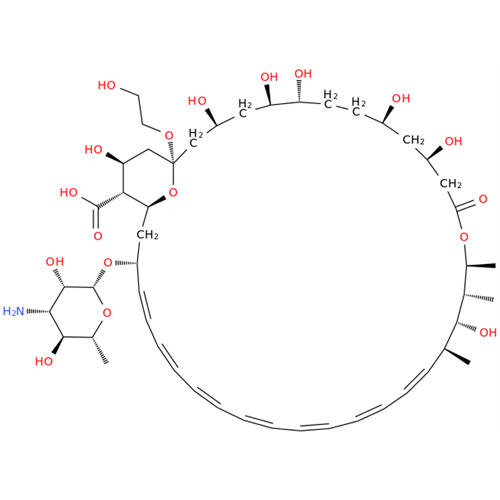
Amphotericin B EP Impurity C
|
Chemical Name: Amphotericin B EP Impurity C
Synonym: Amphotericin B, 13-O-(2-hydroxyethyl)| Enter Batch Number | |||