Product Information
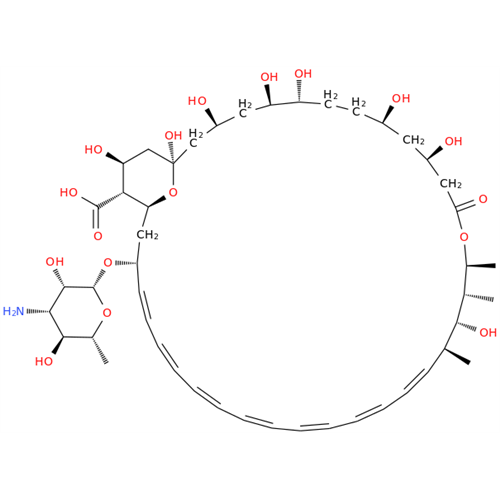
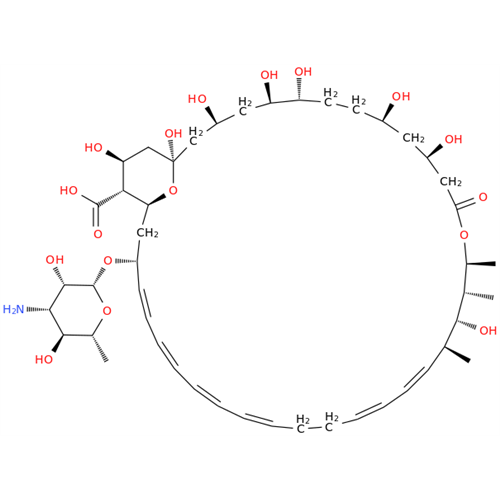
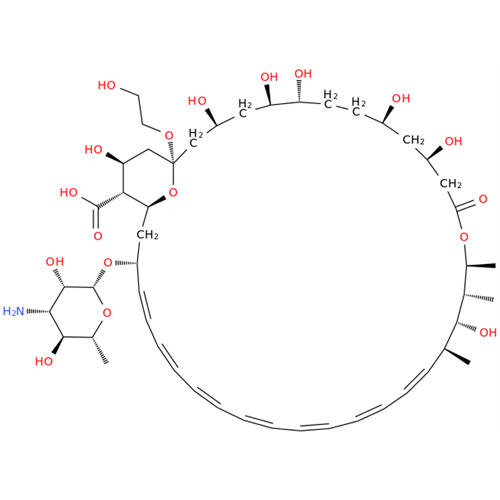
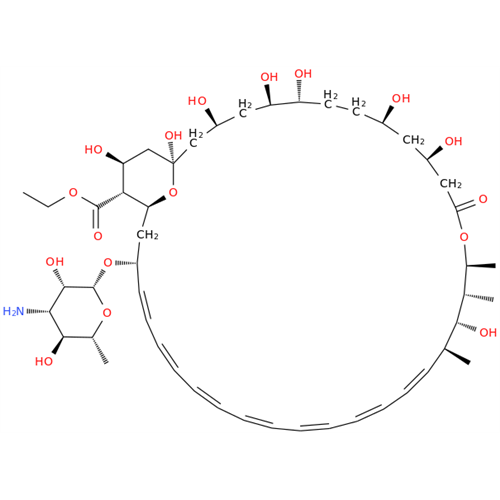
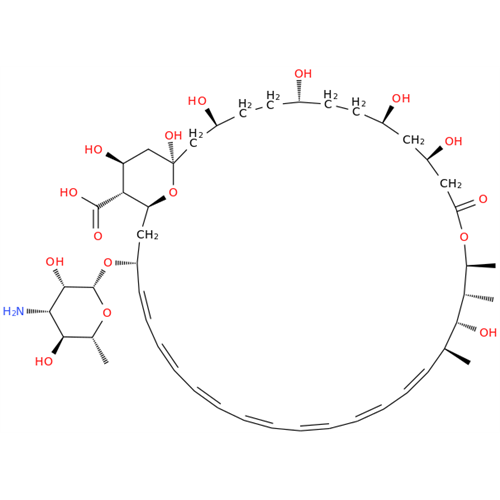
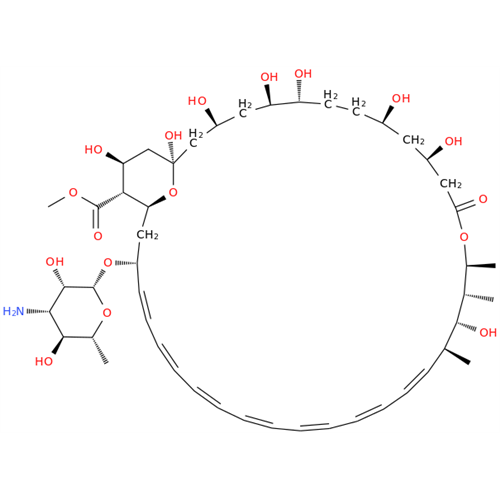
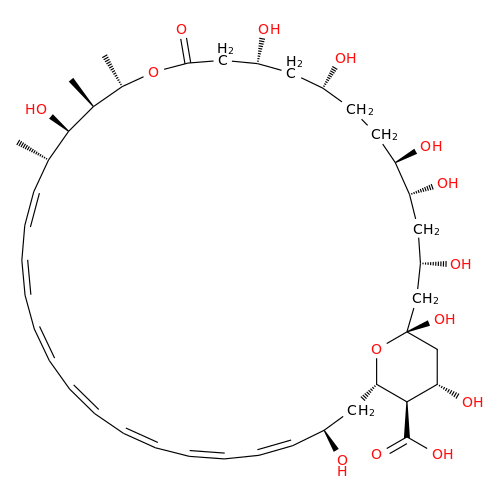
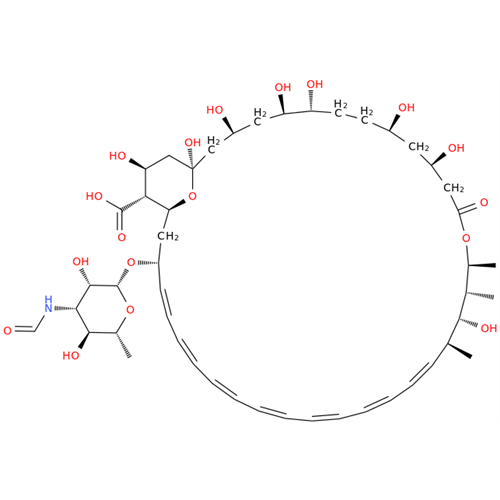
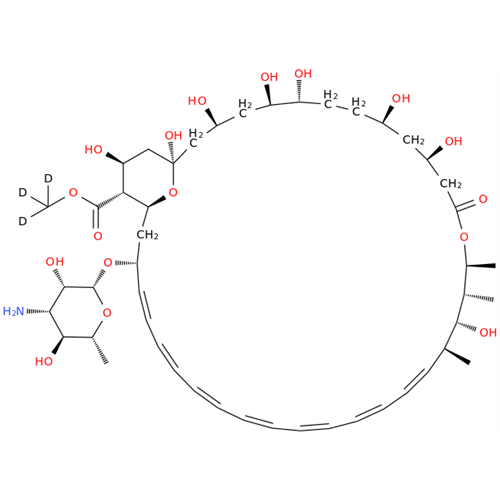
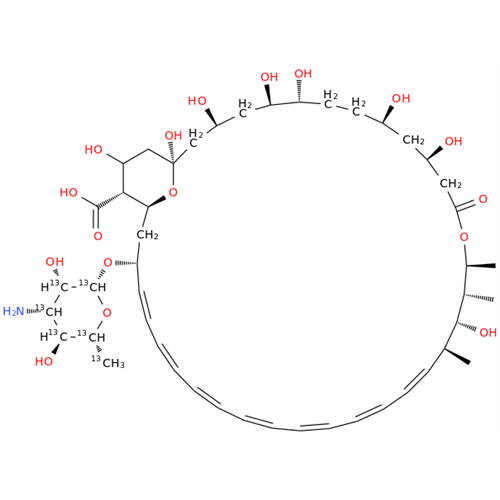
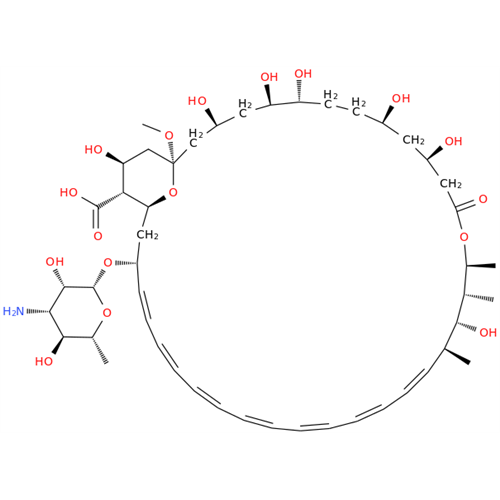
Amphotericin B EP Impurity B
|
Chemical Name: Amphotericin B EP Impurity B
Synonym: Amphotericin Impurity B (EP/BP); 13-O-Methyl-amphotericin B; amphotericin X1| Enter Batch Number | |||