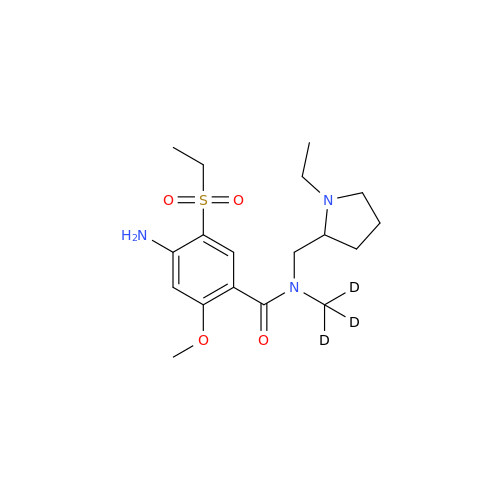
Product Information
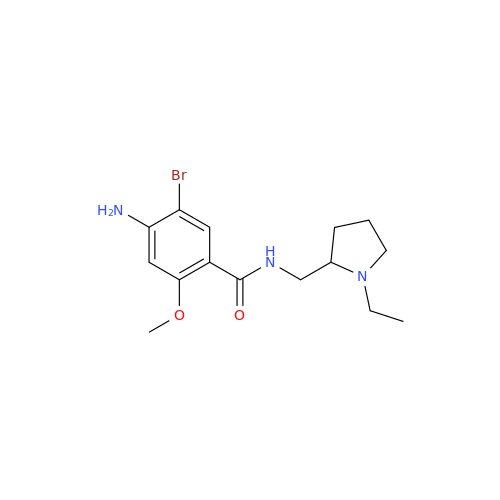
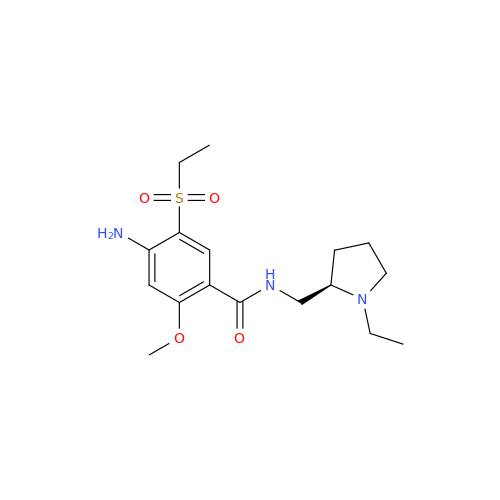
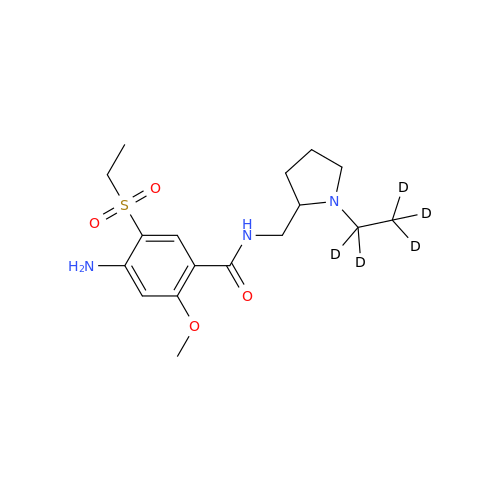
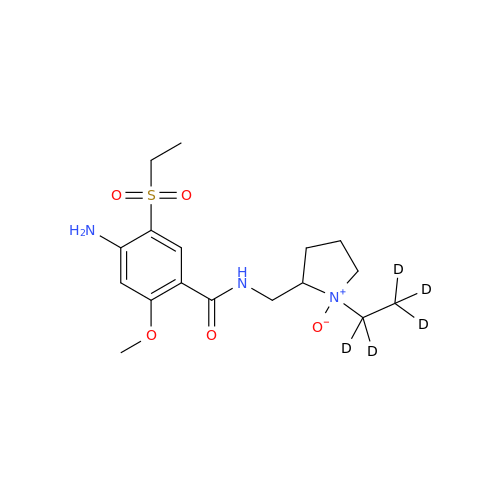
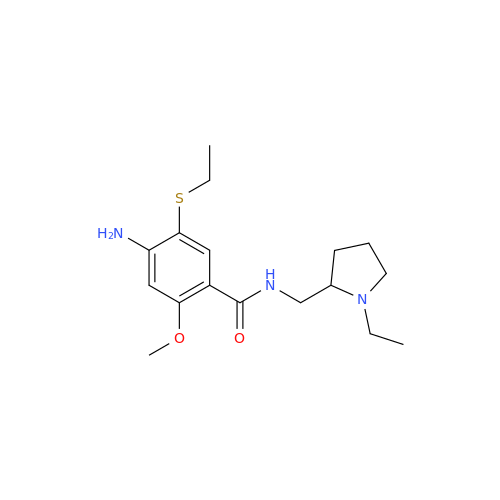
S-Ethyl Amisulpride Impurity
|
Chemical Name: S-Ethyl Amisulpride Impurity
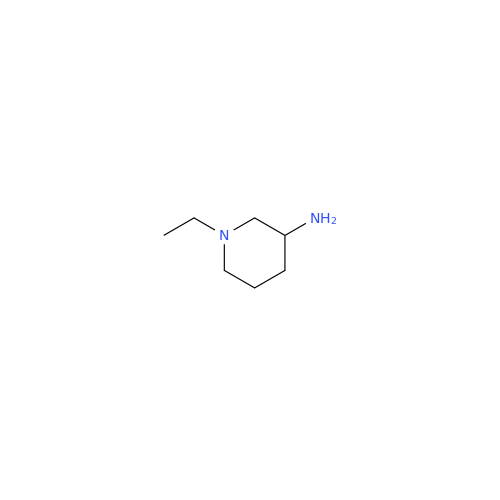
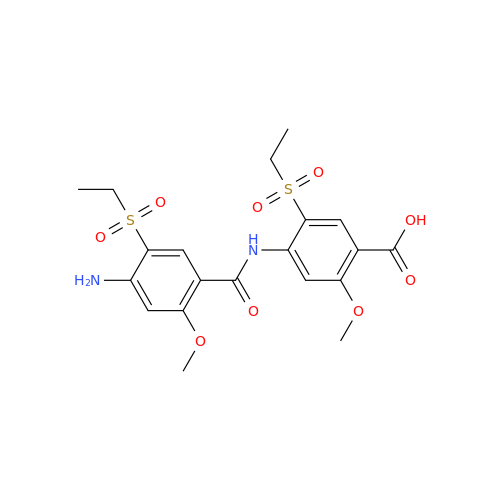
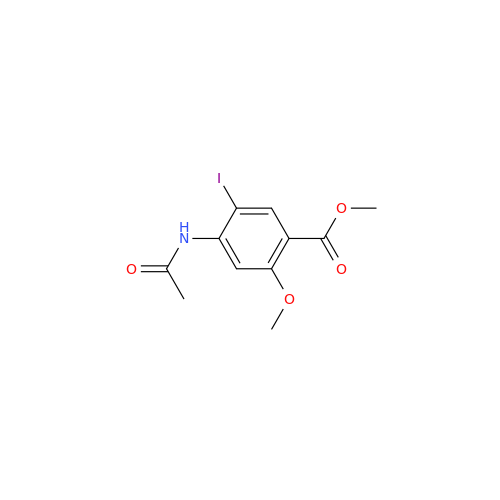
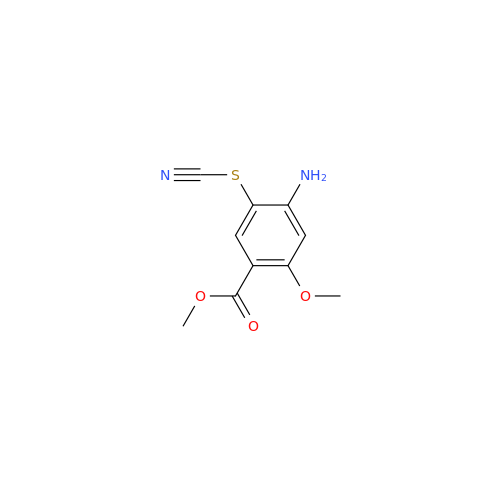
Synonym: 4-Amino-N-((1-ethylpyrrolidin-2-yl)methyl)-5-(ethylthio)-2-methoxybenzamide| Enter Batch Number | |||



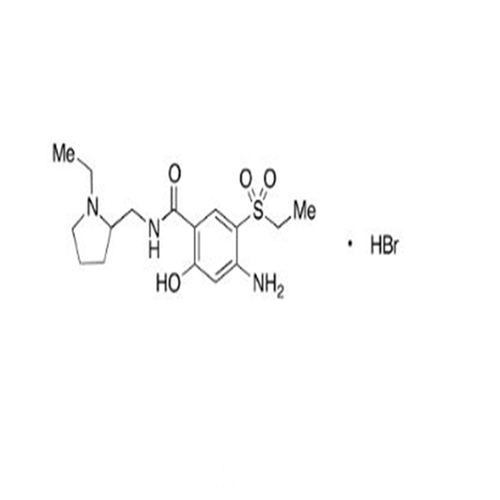
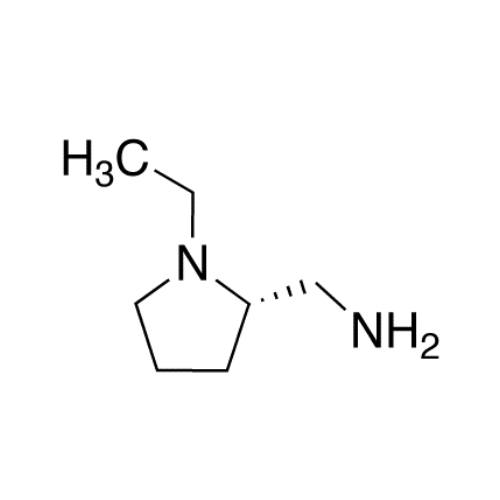
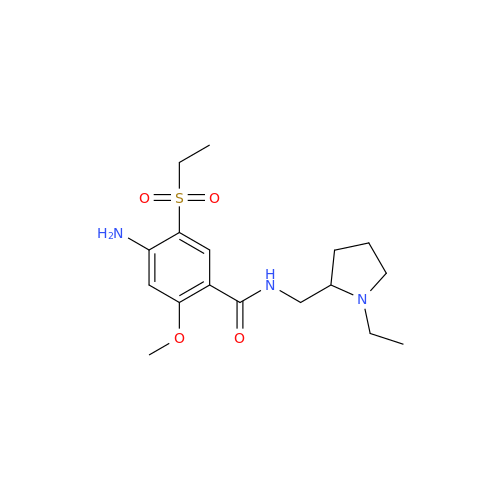
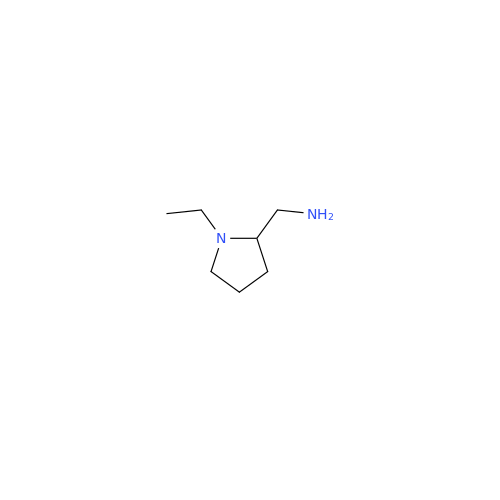
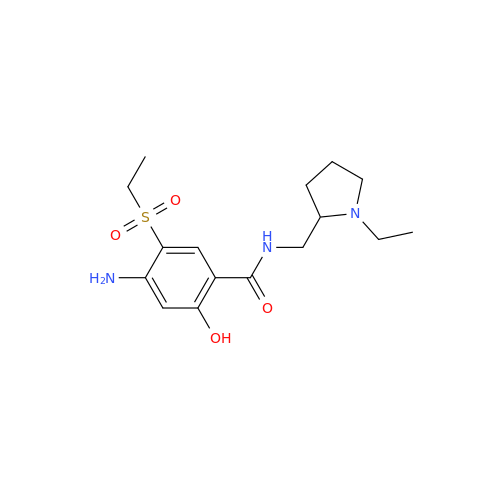
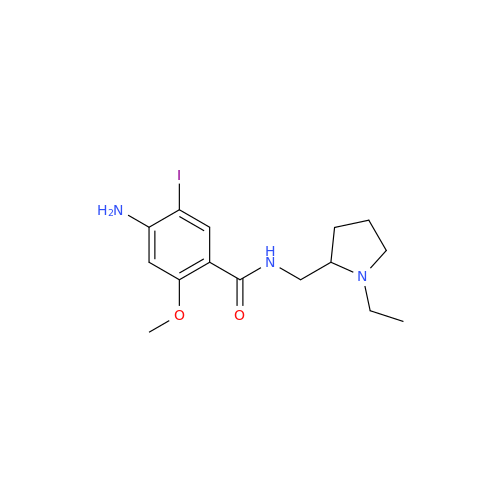
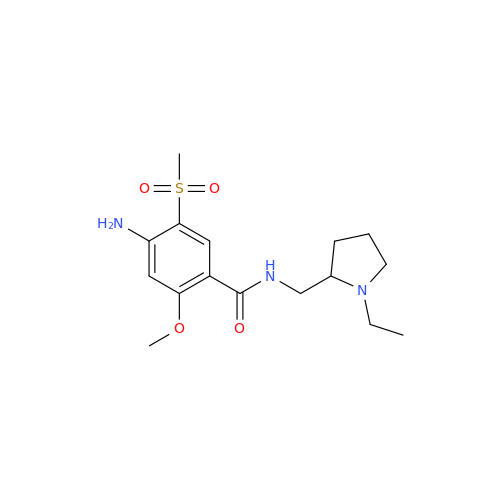
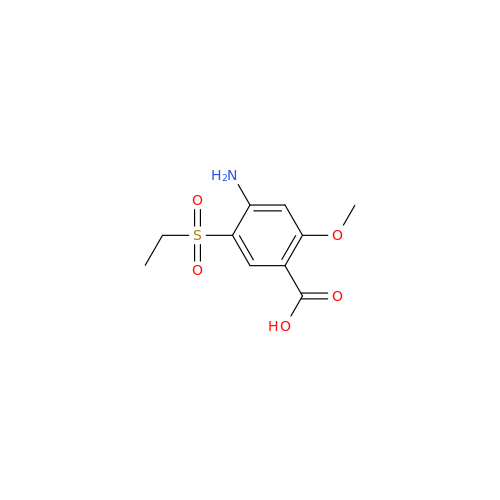
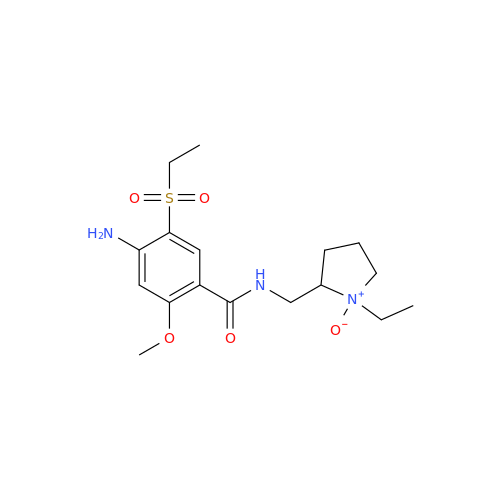
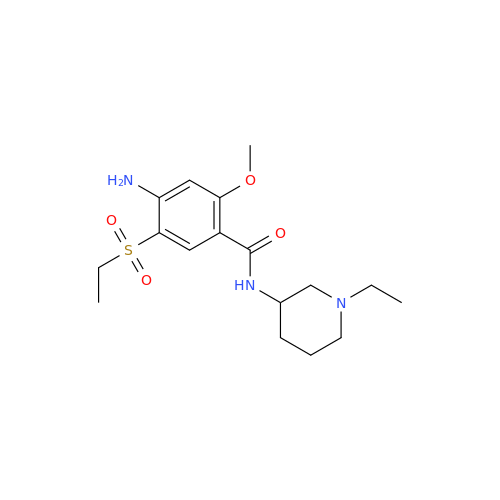
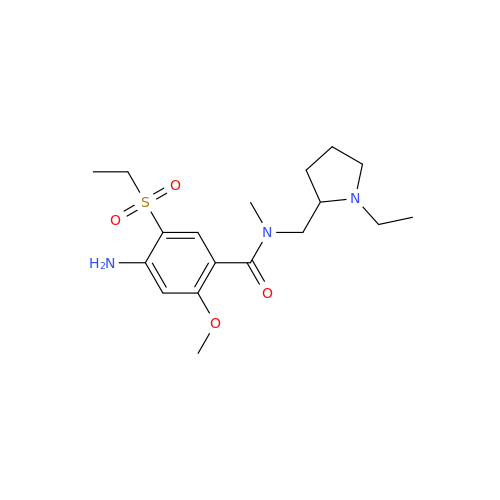
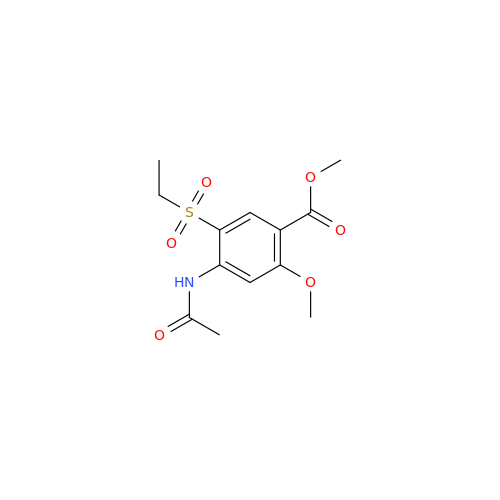
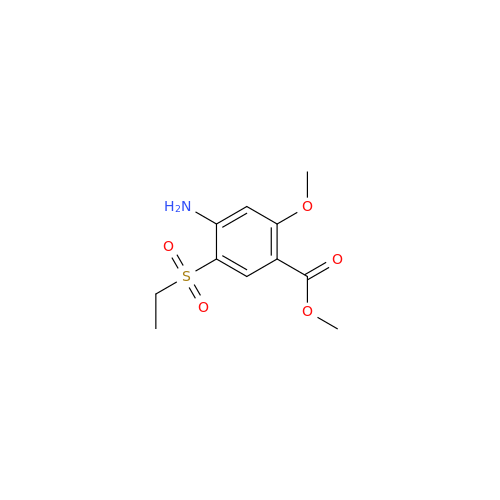
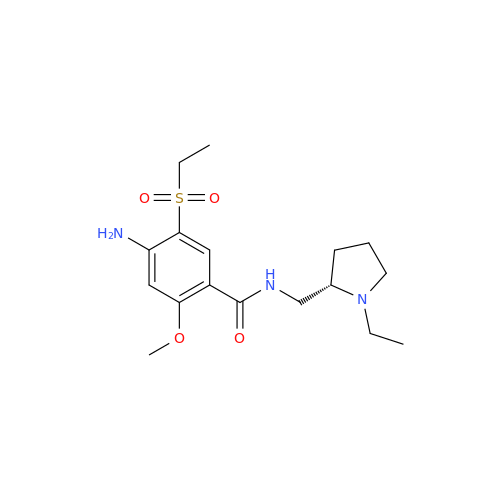
![1-Ethyl-2-[(methylamino)methyl]pyrrolidine 1-Ethyl-2-[(methylamino)methyl]pyrrolidine](/uploads/product-details/ams015-6401.png)