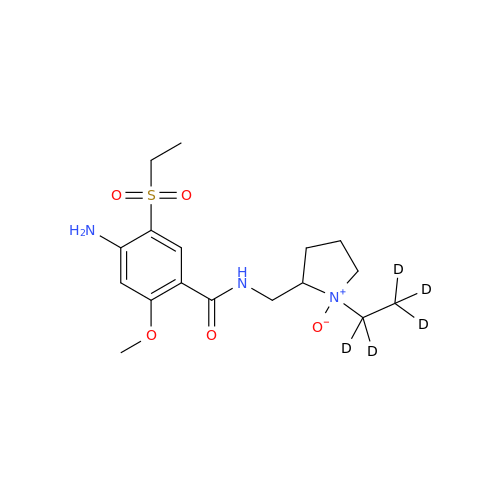
Product Information
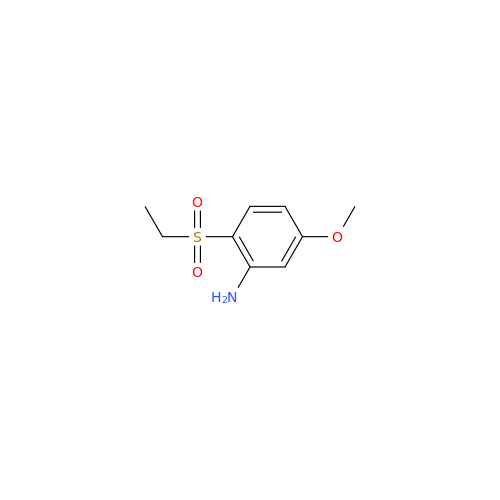
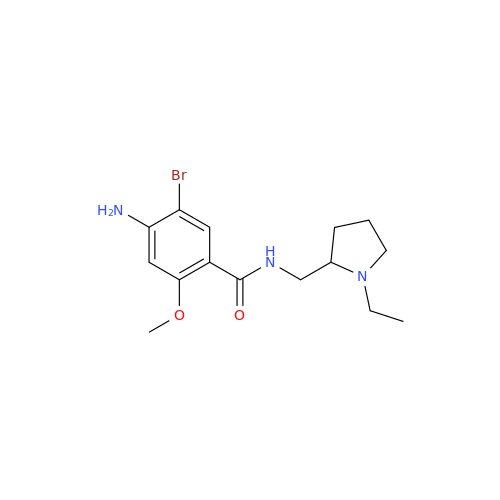
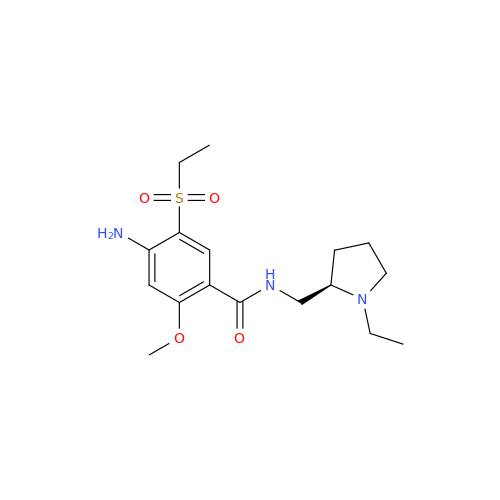
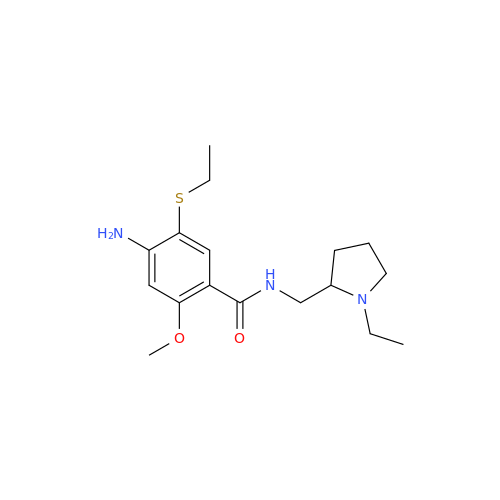
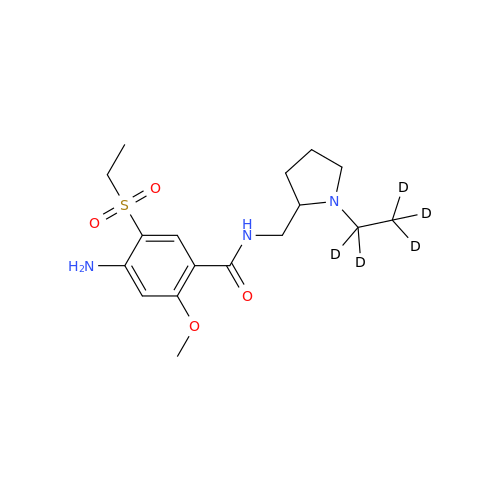
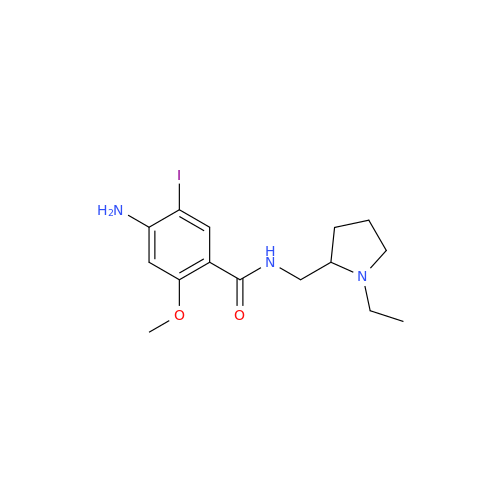
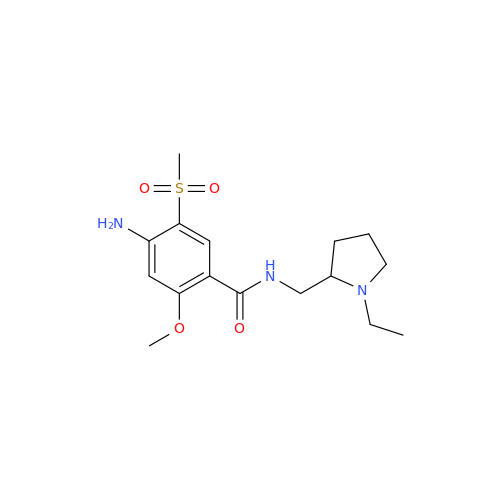
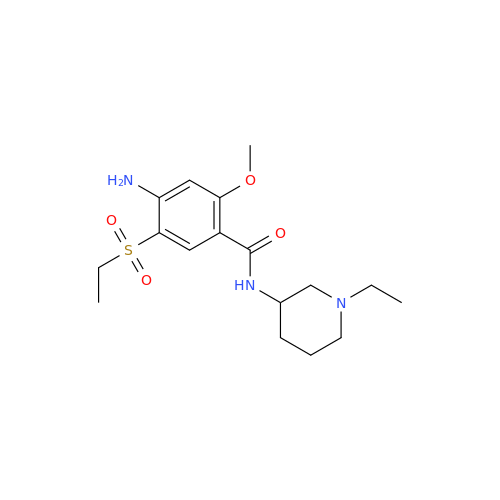
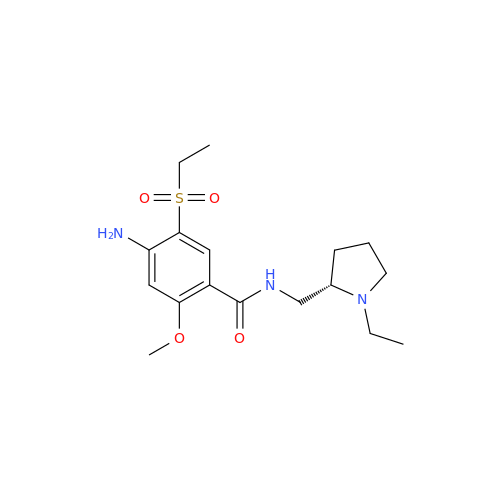
Amisulpride EP Impurity H
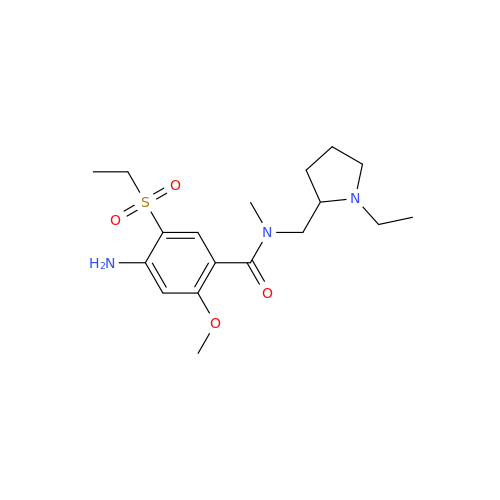
|
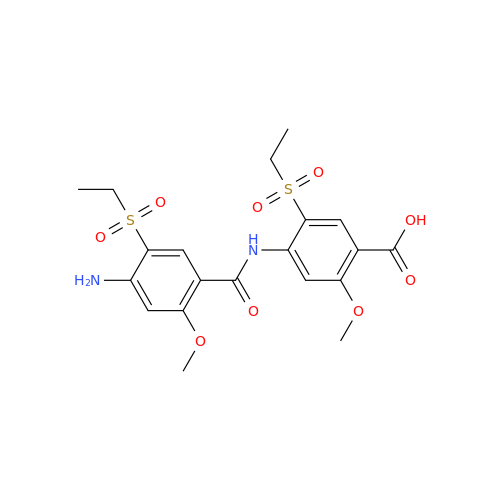
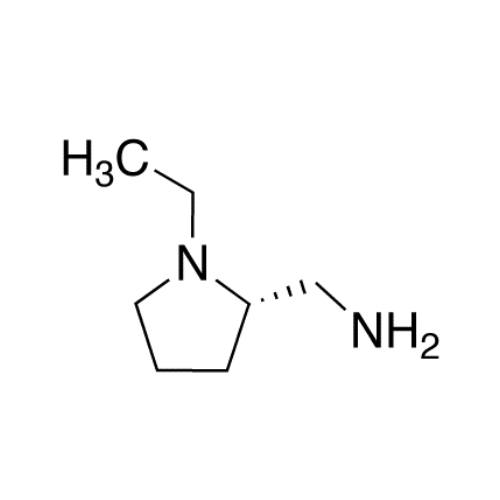
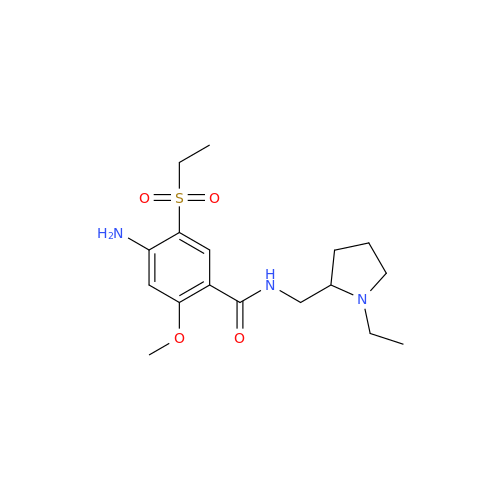
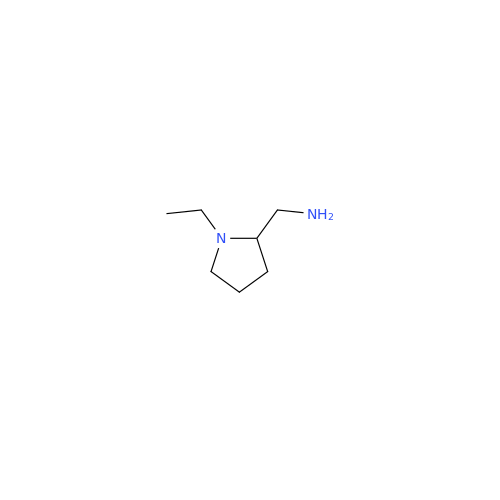
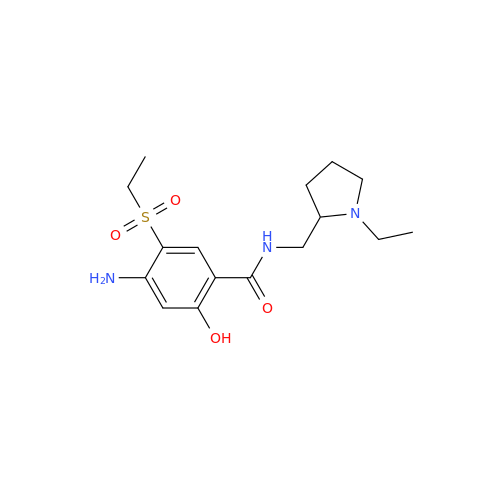
Chemical Name: Amisulpride EP Impurity H
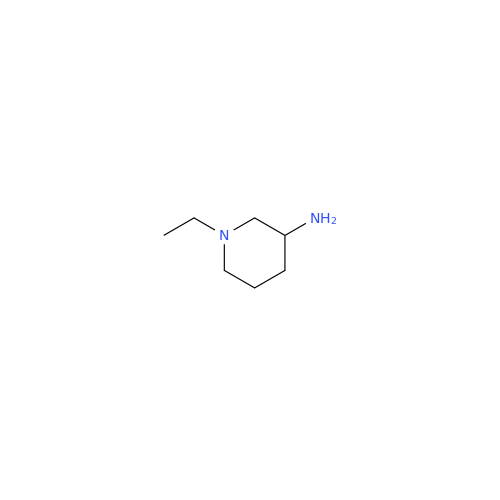
Synonym: 4-Amino-N-[[(2RS)-1-ethylpyrrolidin-2-yl]methyl]-5-(ethylsulfonyl)-2-methoxy-N-methylbenzamide| Enter Batch Number | |||



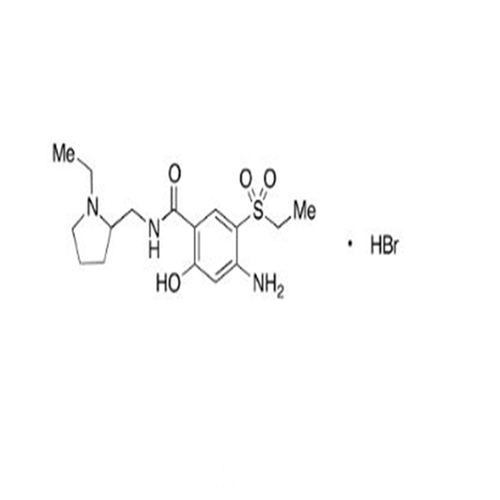
![1-Ethyl-2-[(methylamino)methyl]pyrrolidine 1-Ethyl-2-[(methylamino)methyl]pyrrolidine](/uploads/product-details/ams015-6401.png)