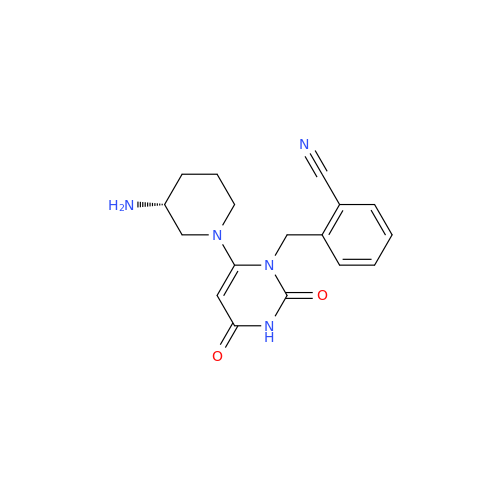
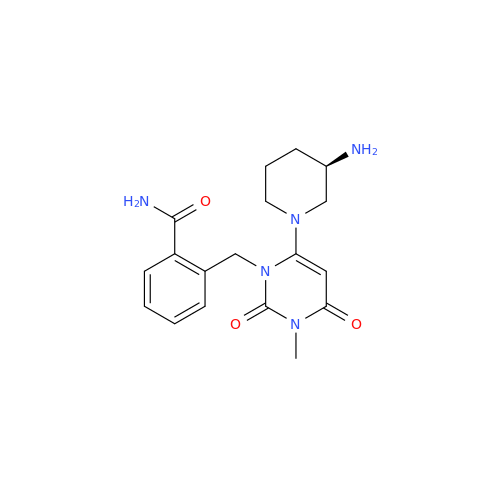
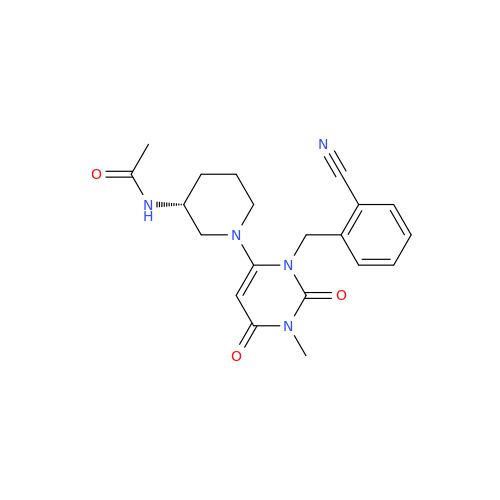
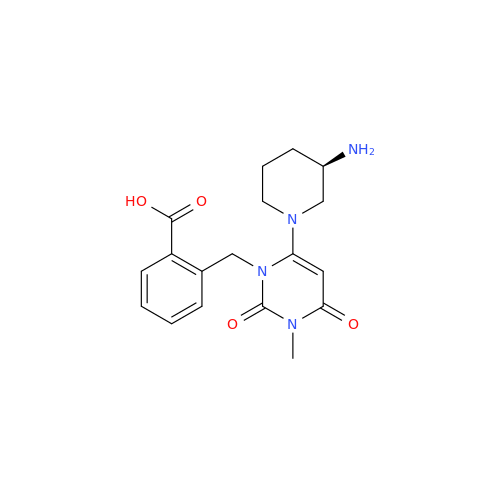
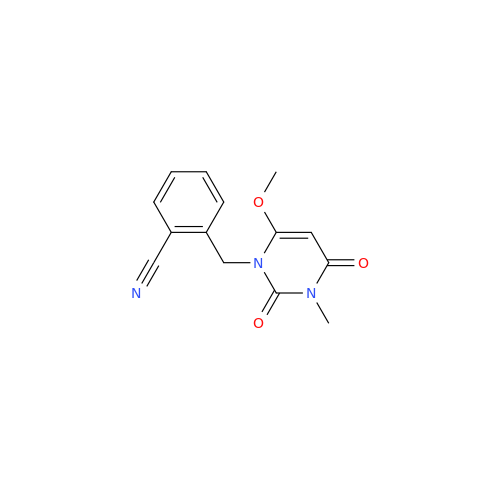
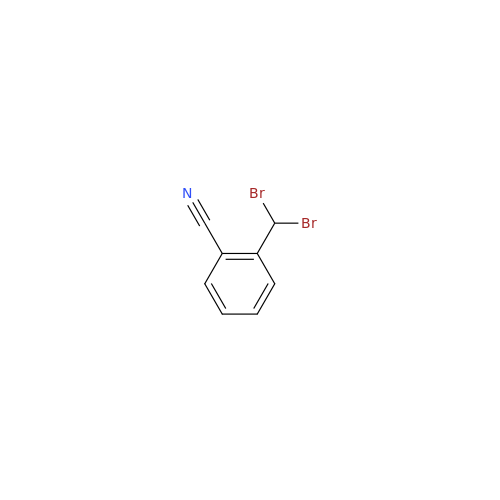
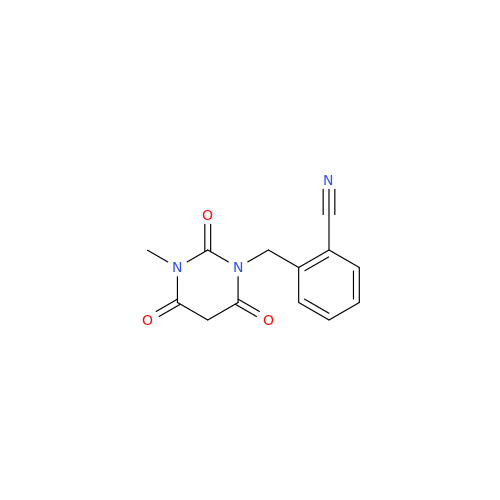
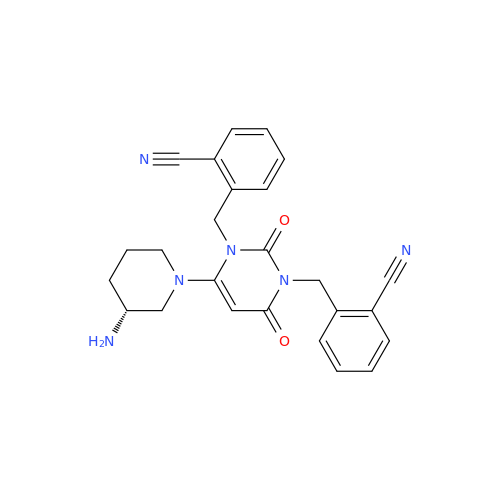
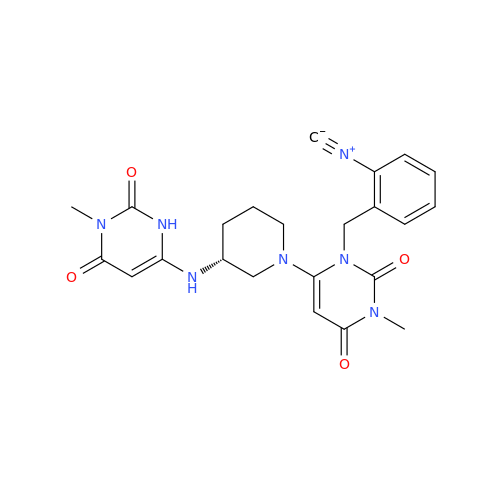
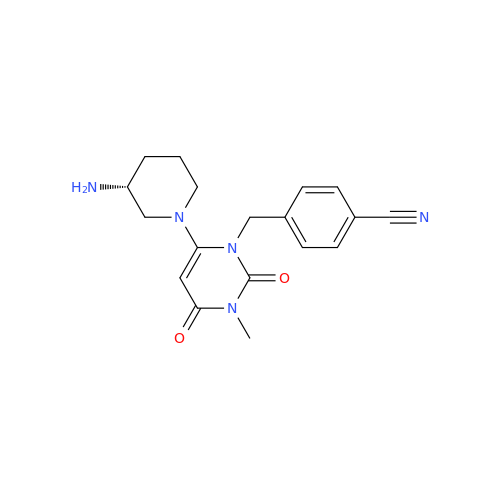
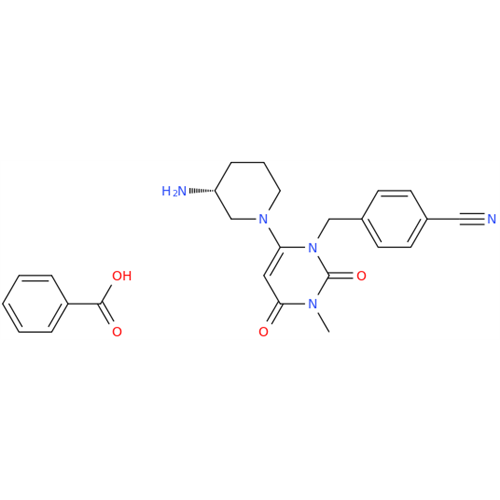
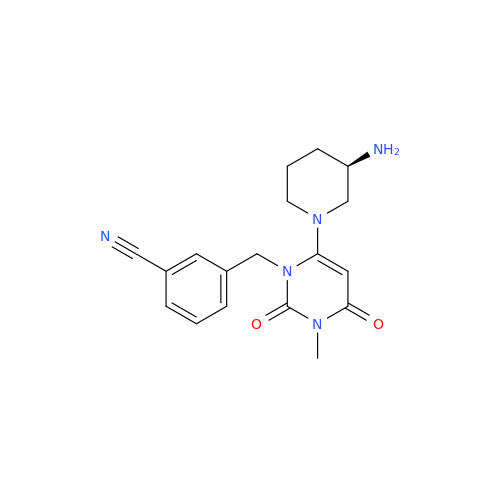
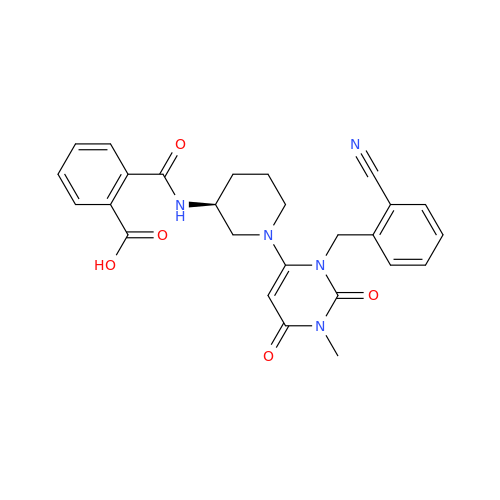
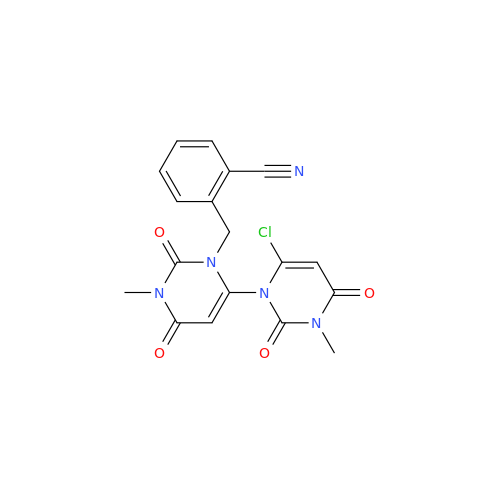
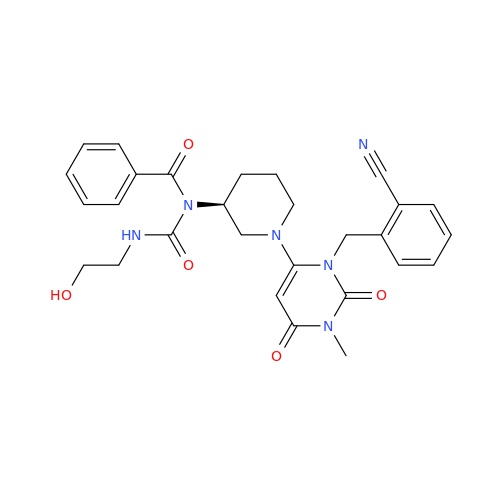
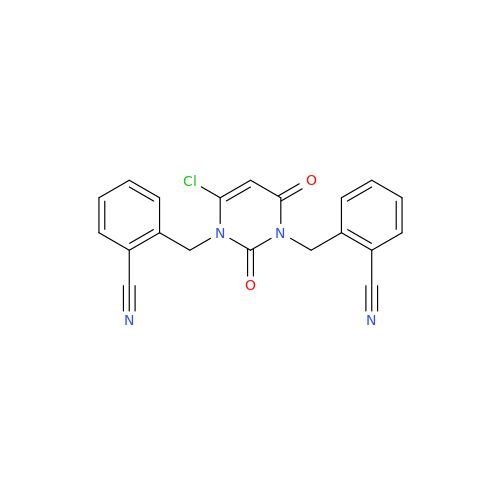
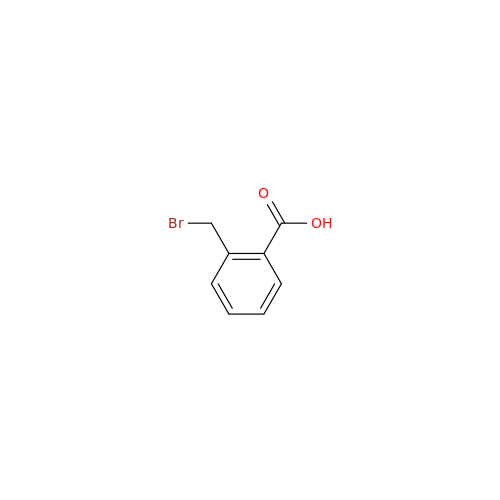
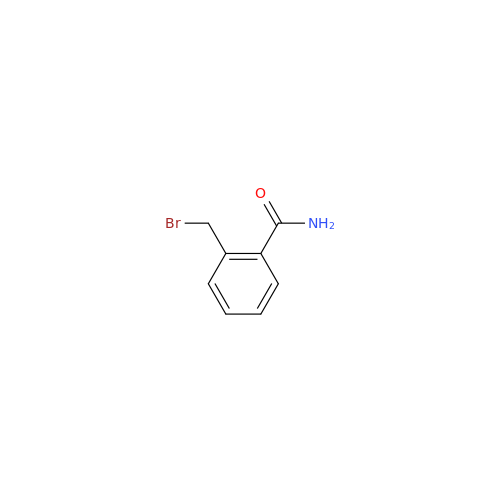
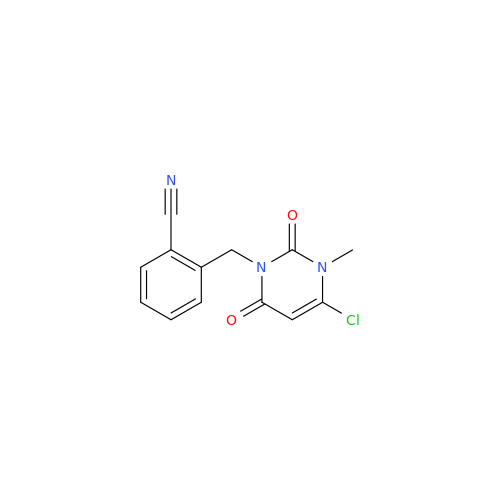
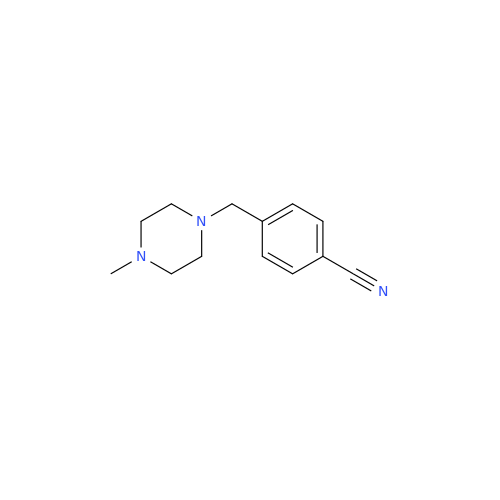
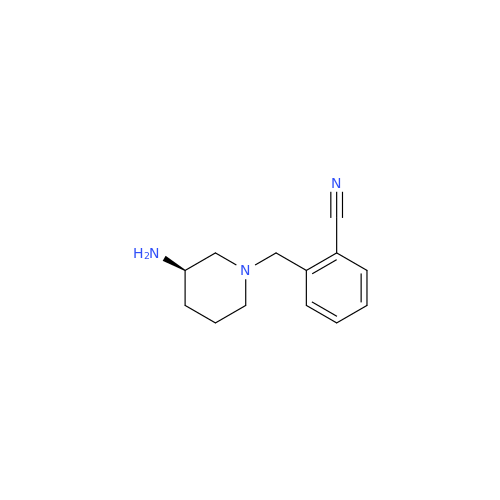
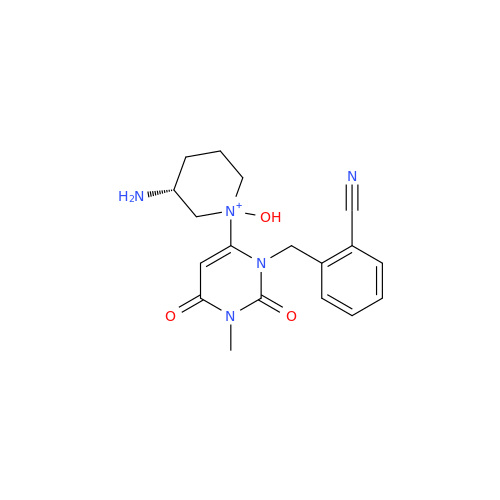
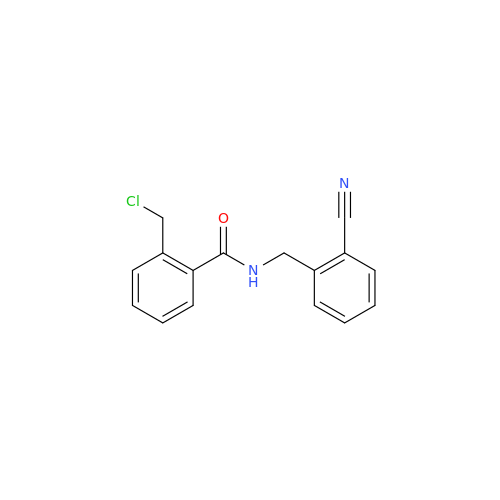
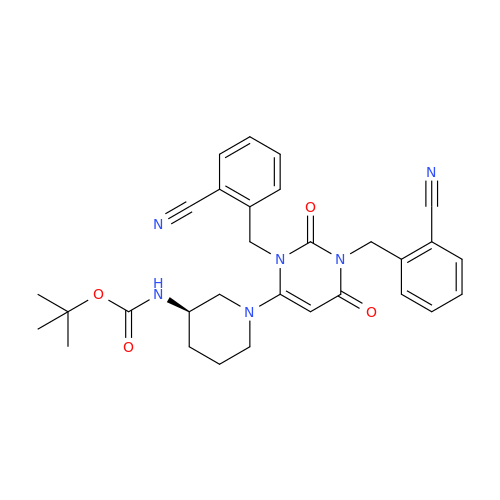
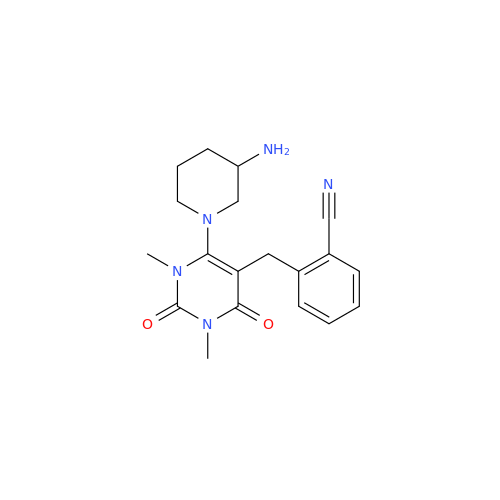
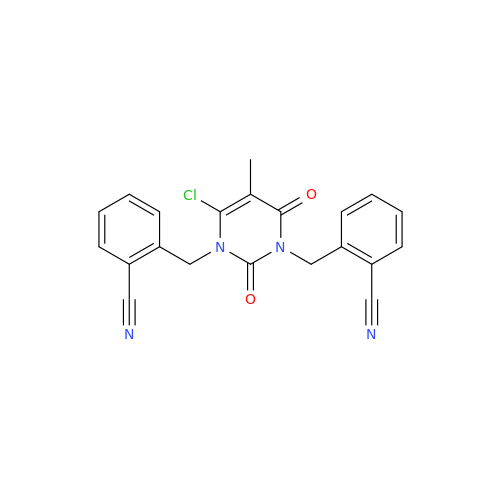
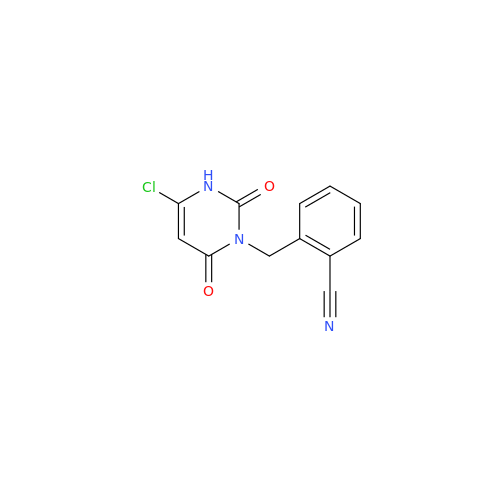
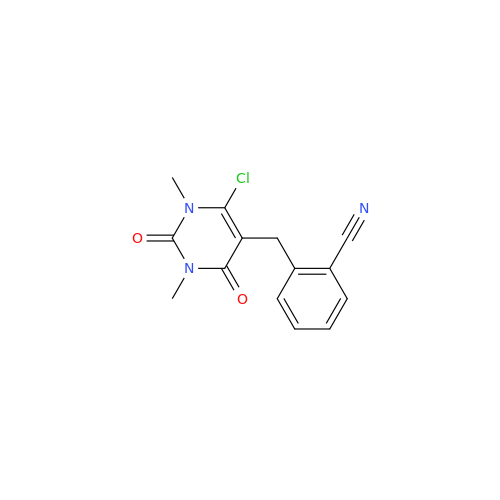
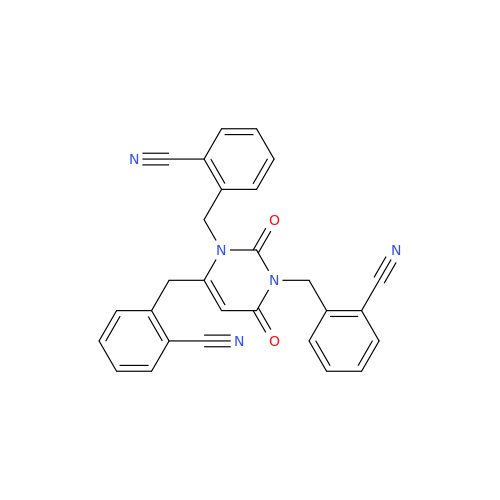
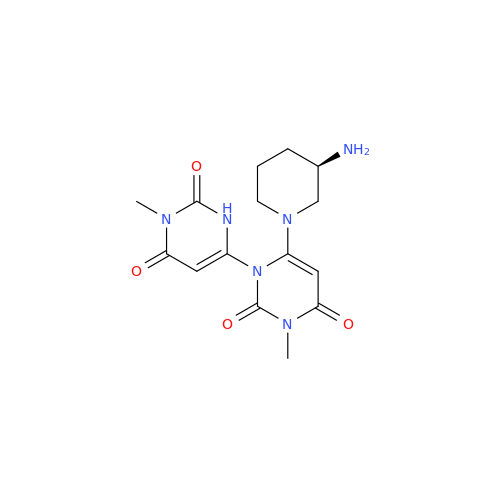
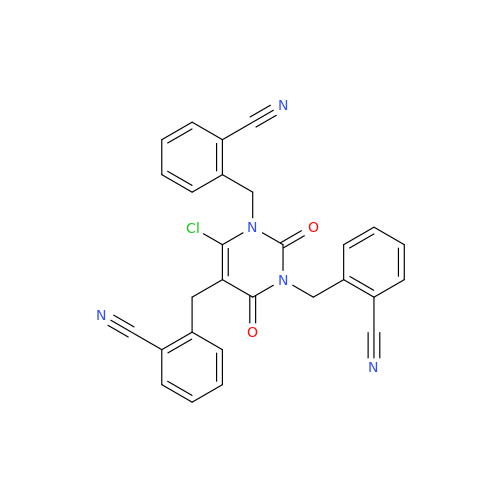
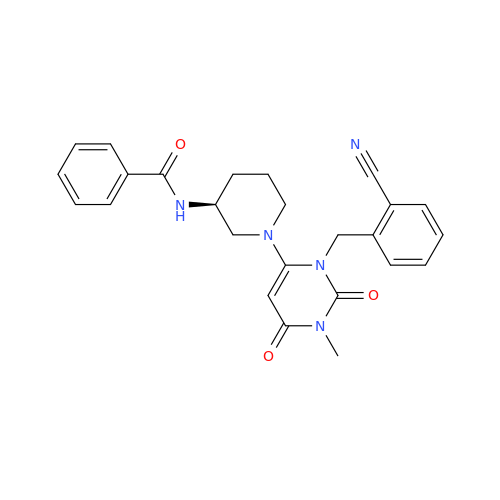
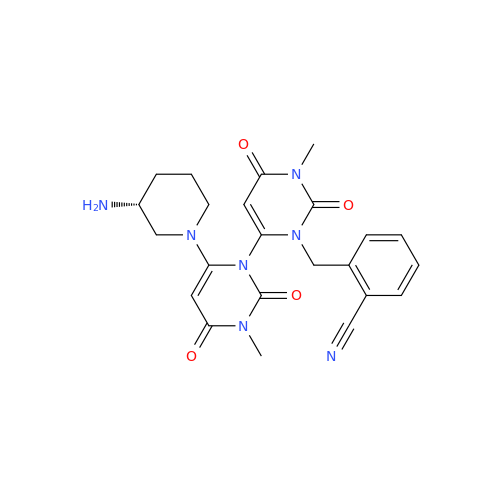
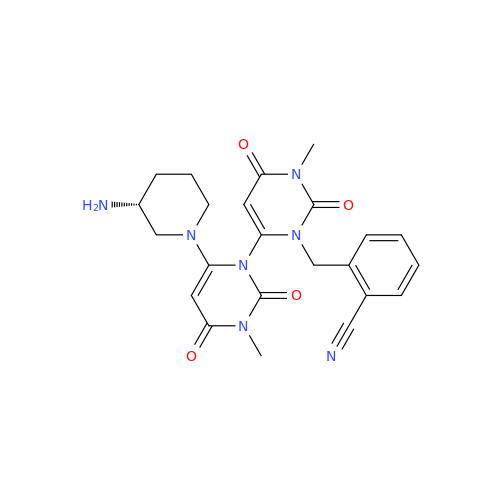
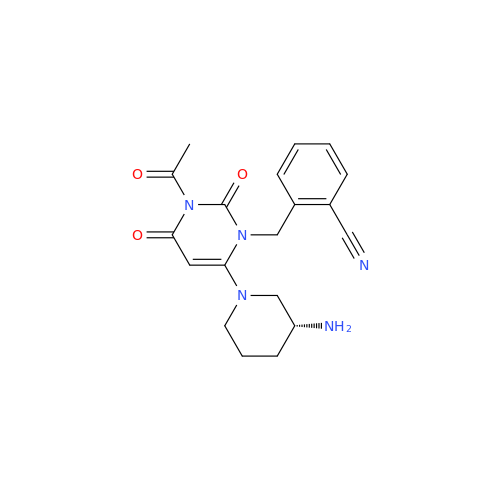
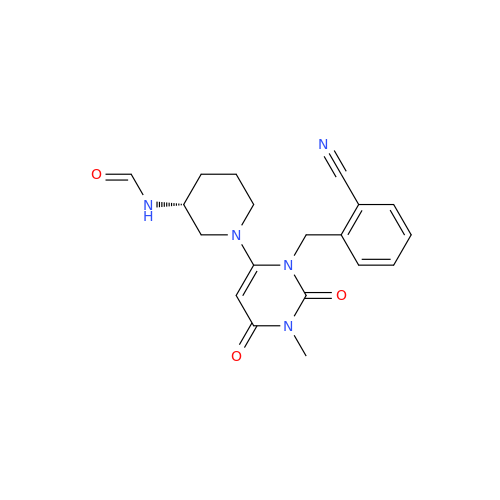
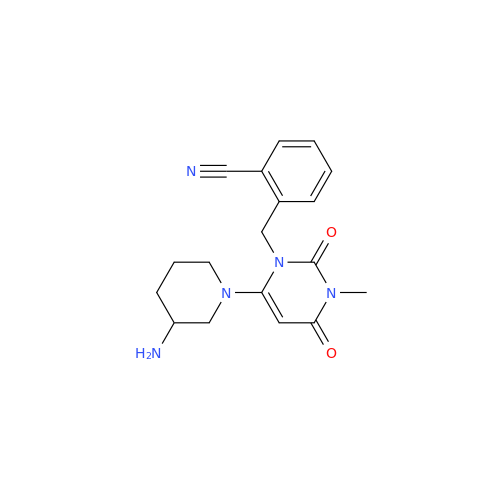
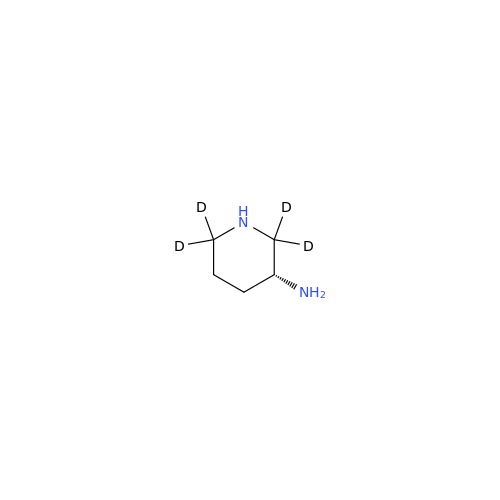
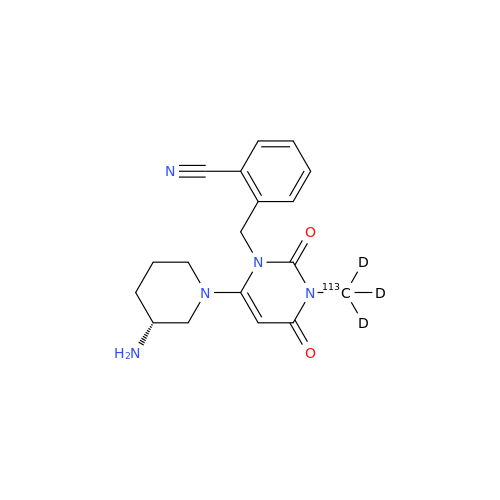
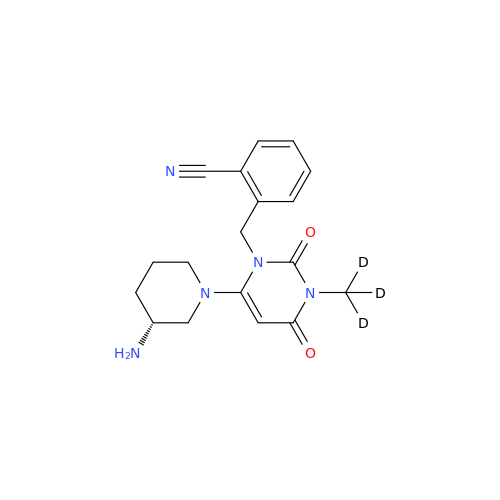
Product Information
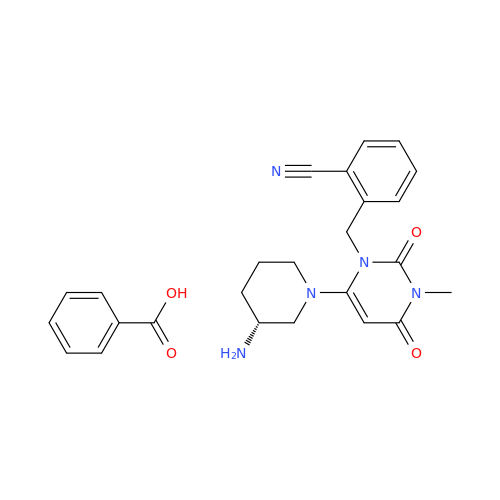
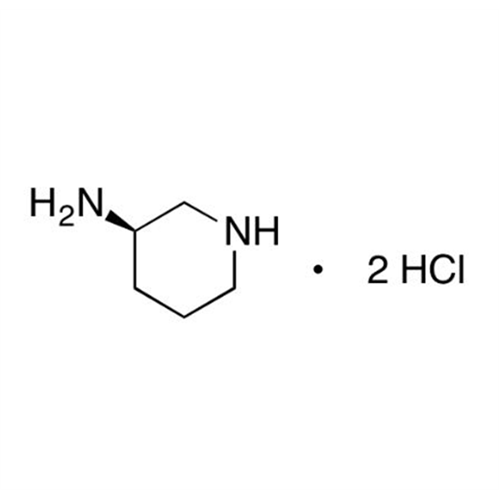
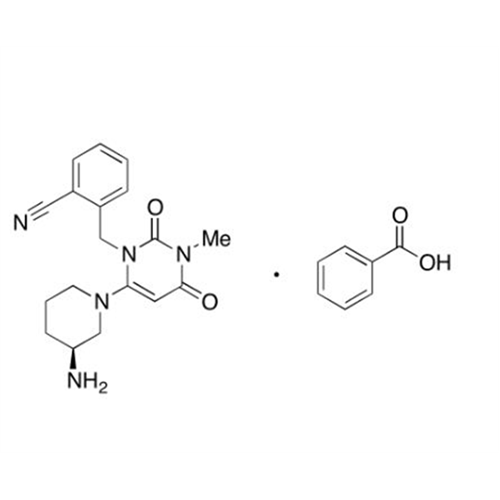
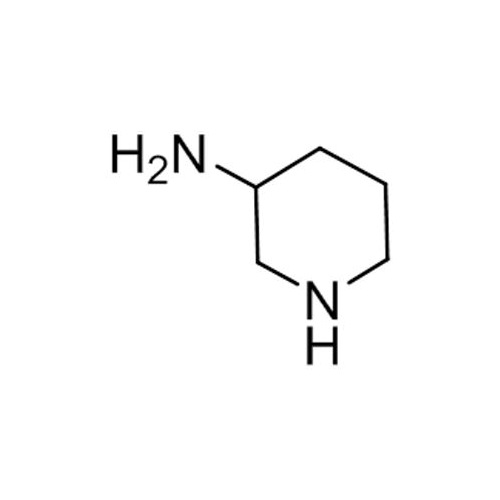
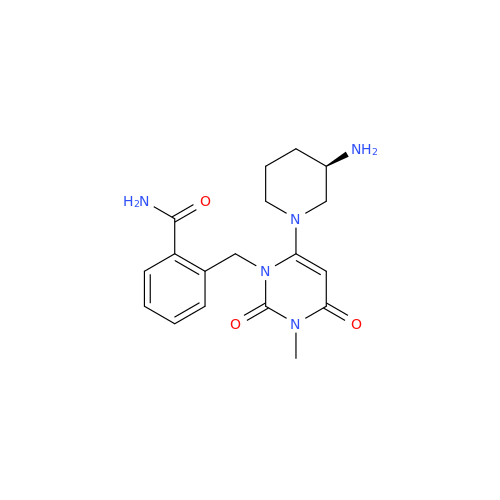
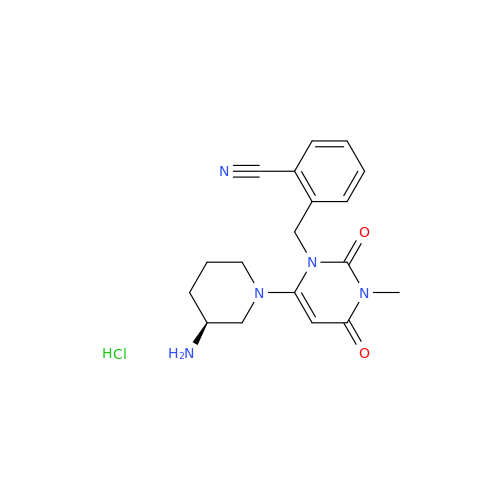
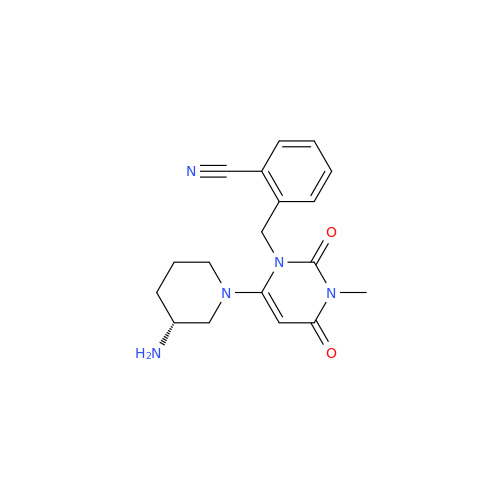
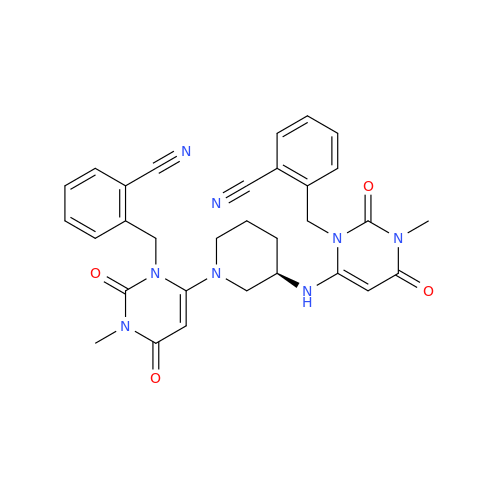
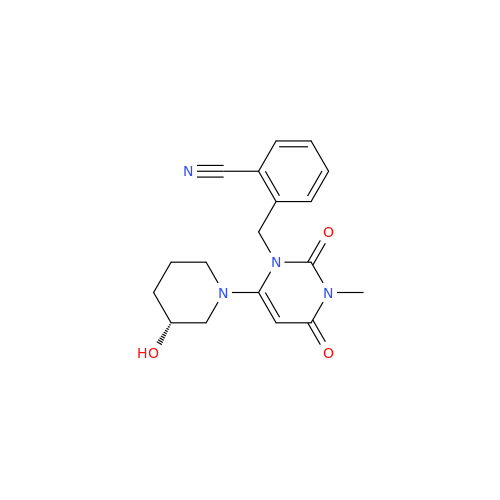
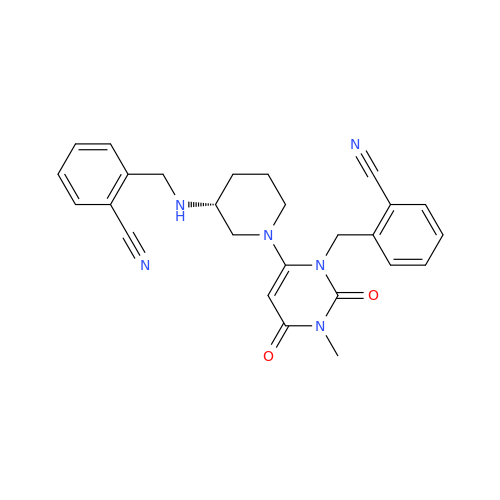
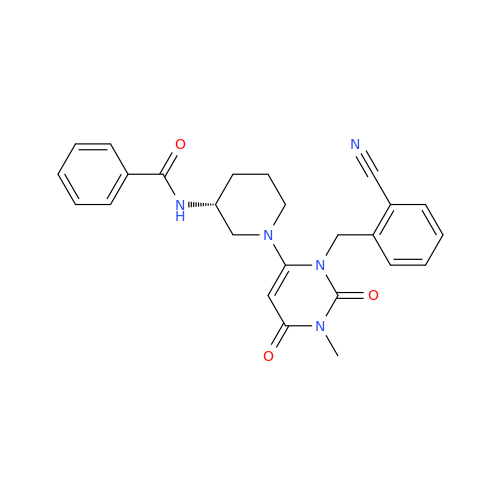
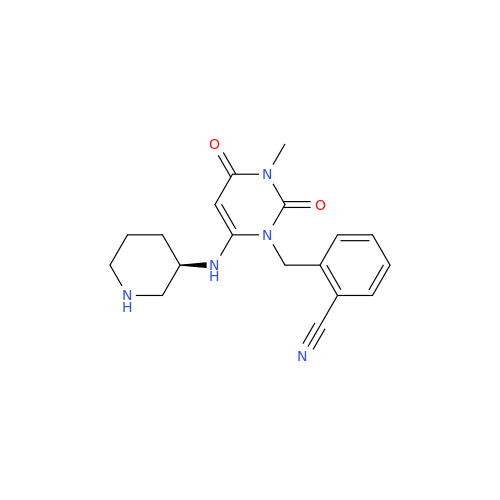
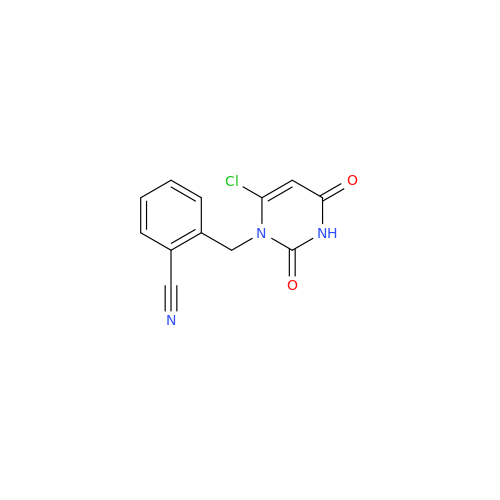
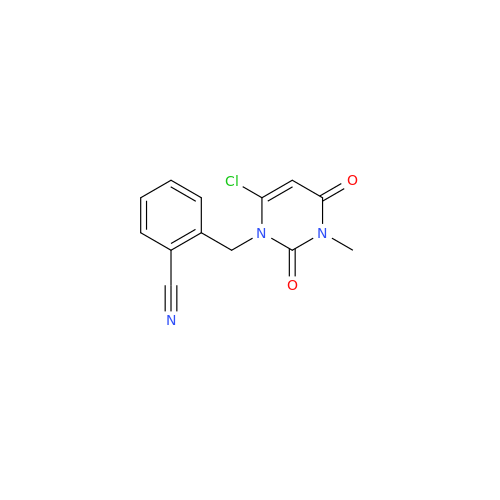
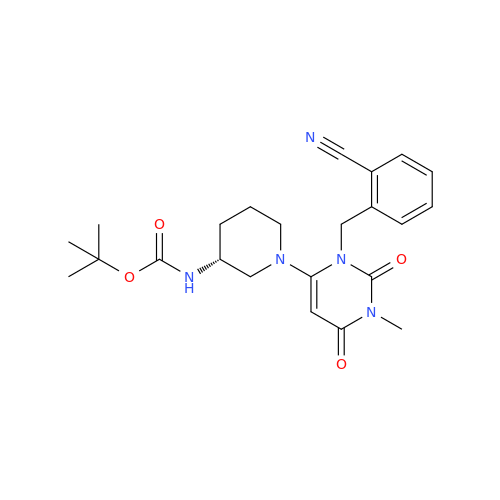
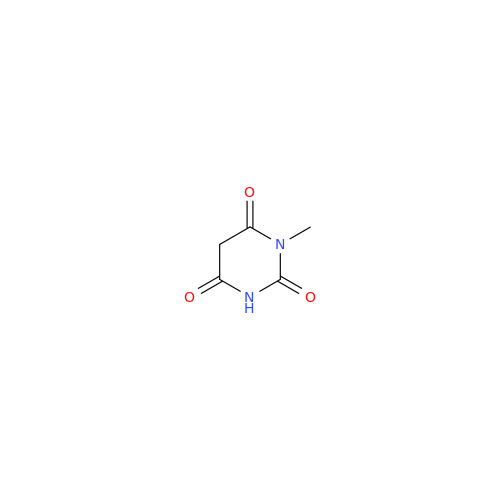
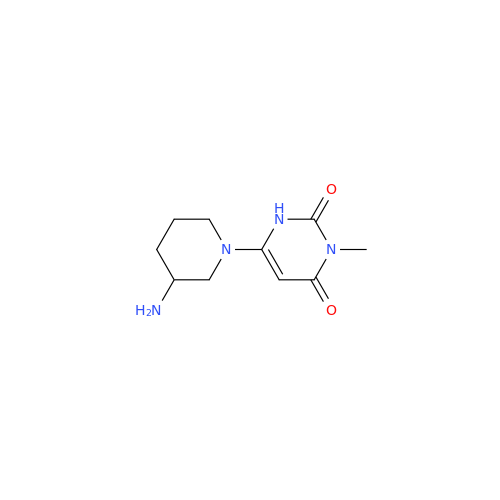
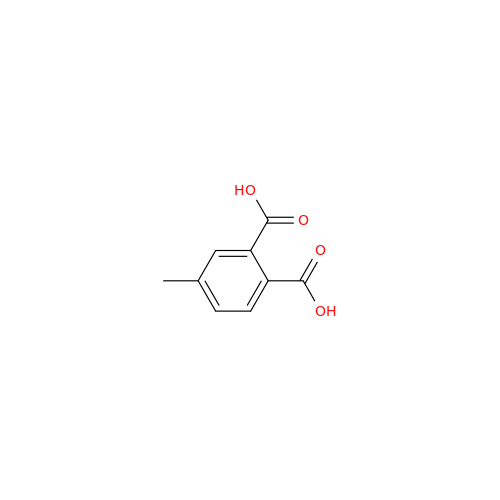
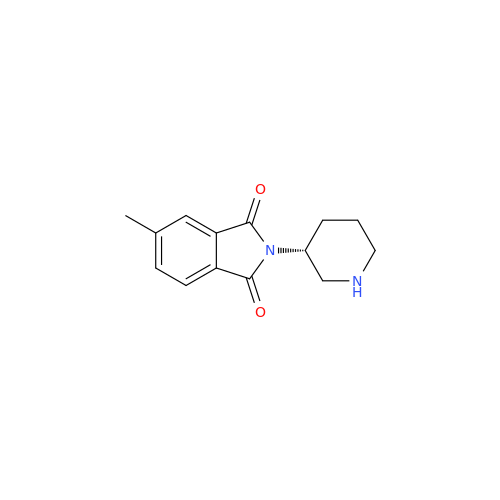
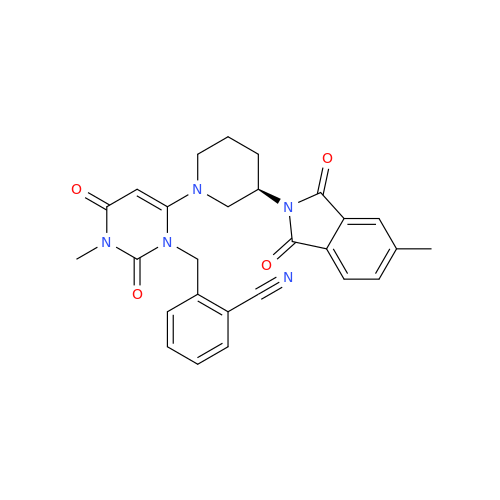
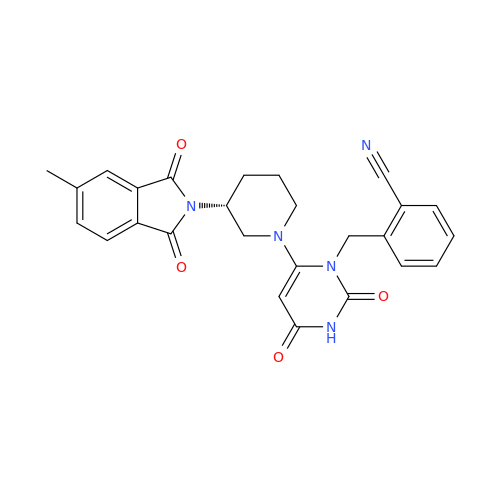
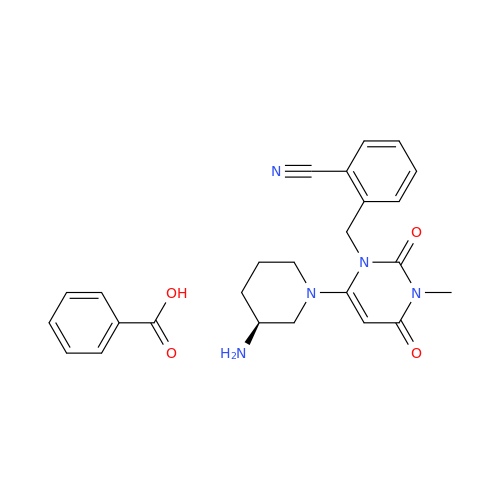
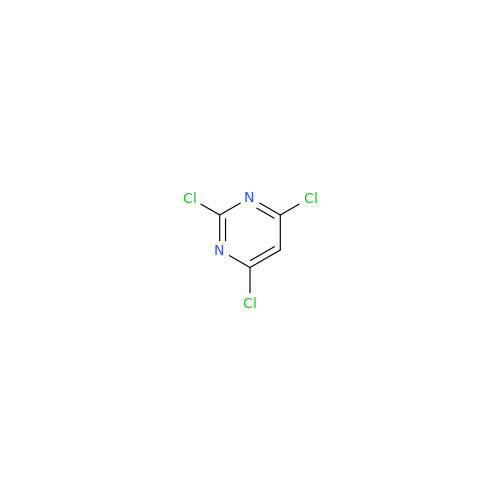
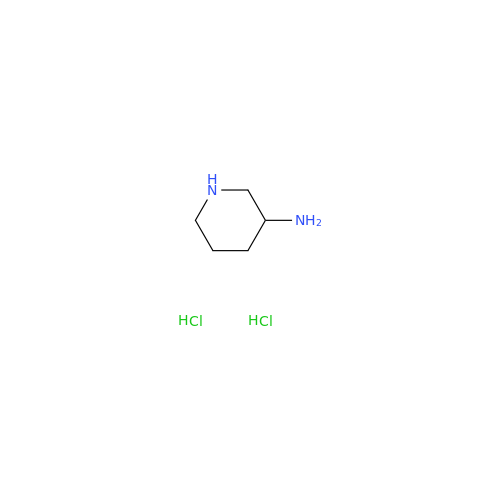
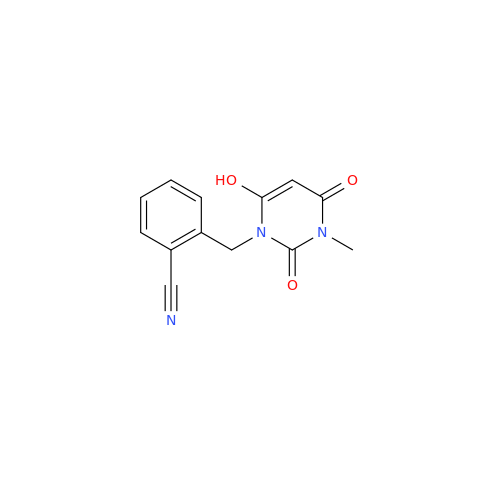
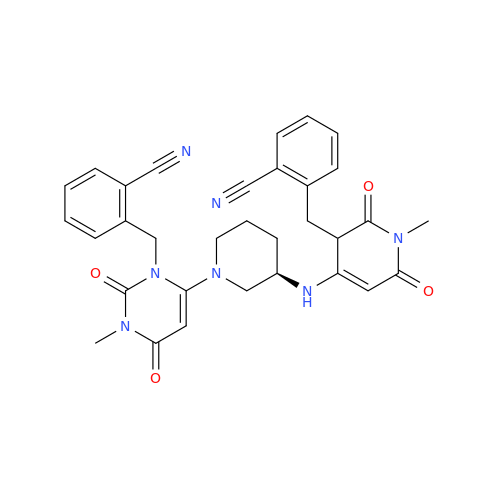
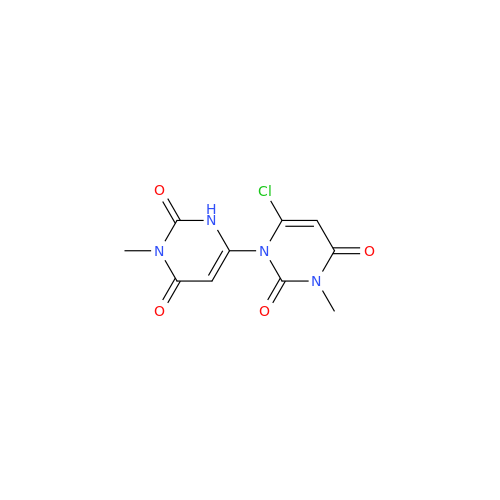
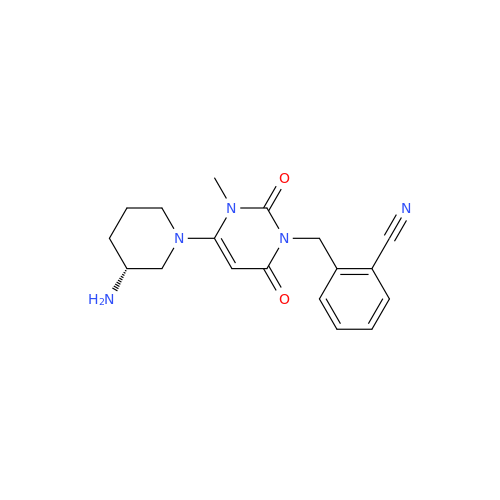
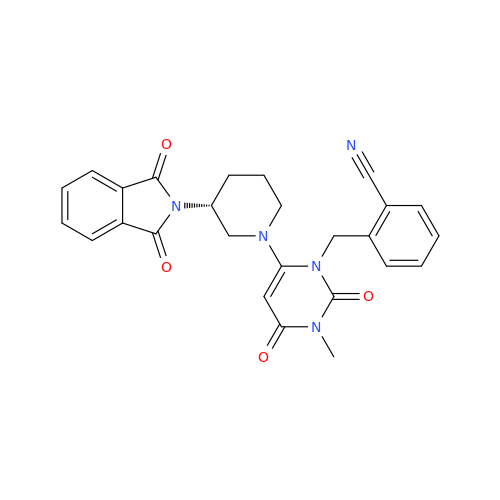
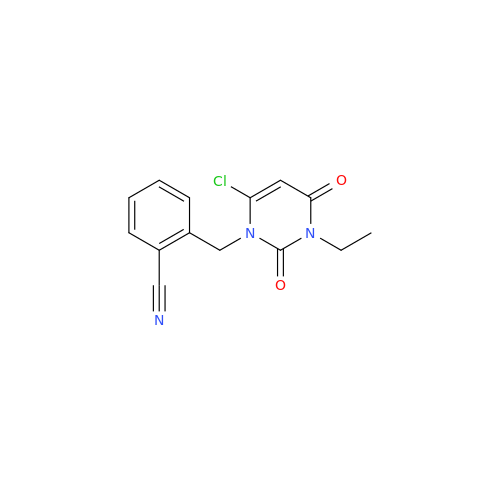
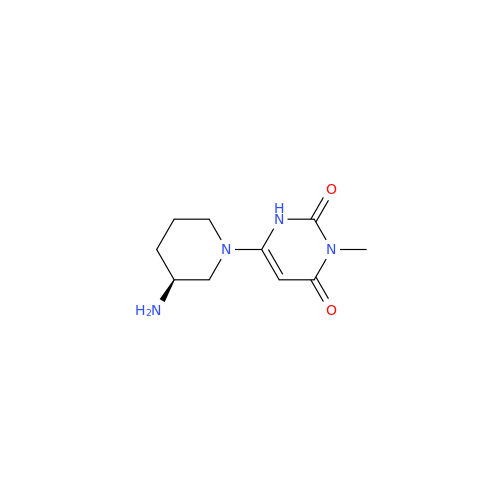
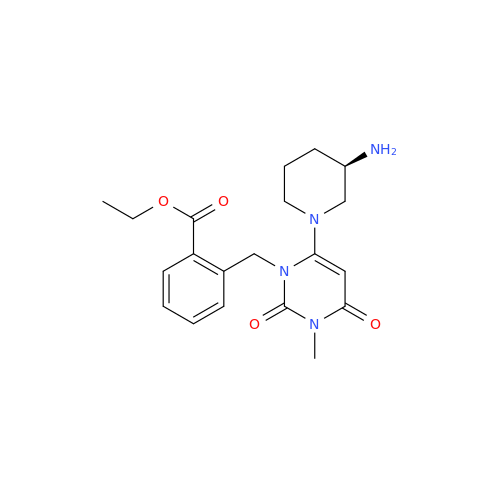
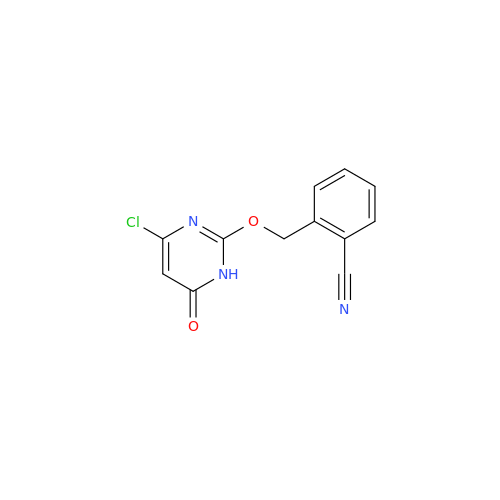
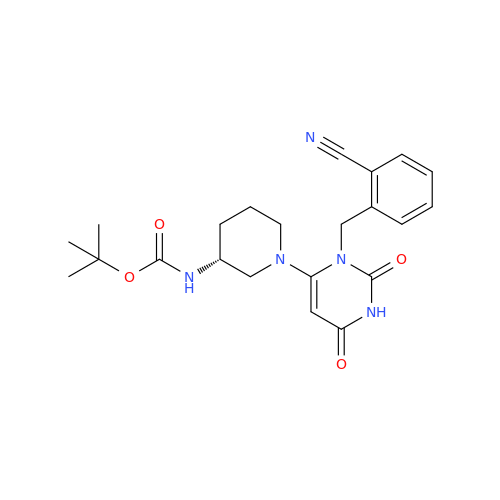
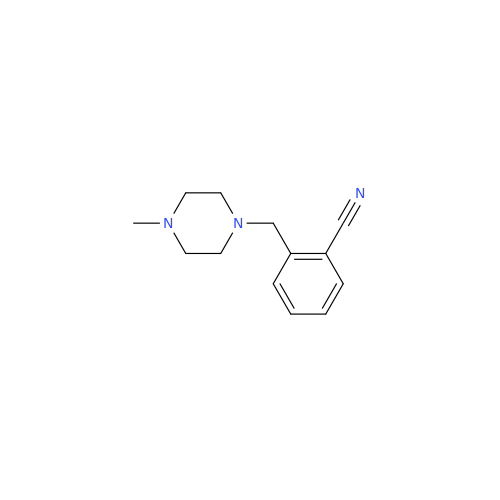
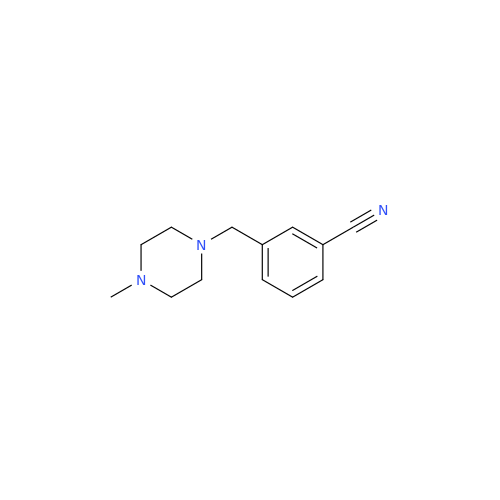
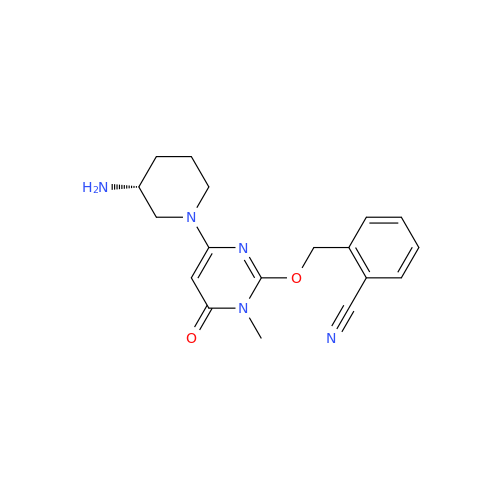
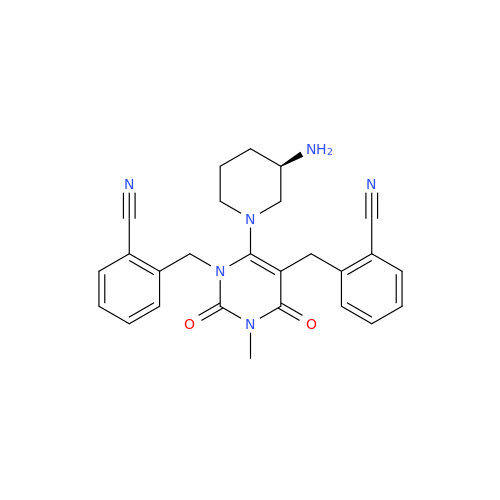
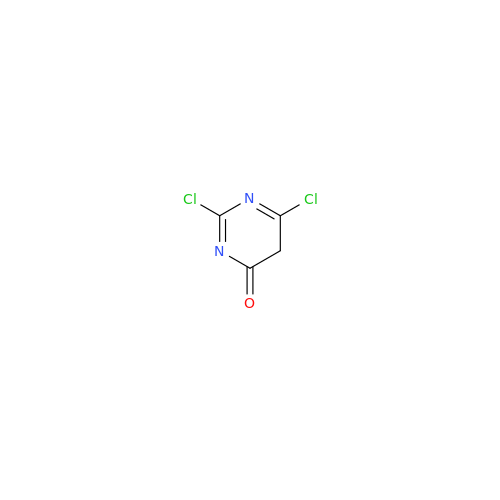
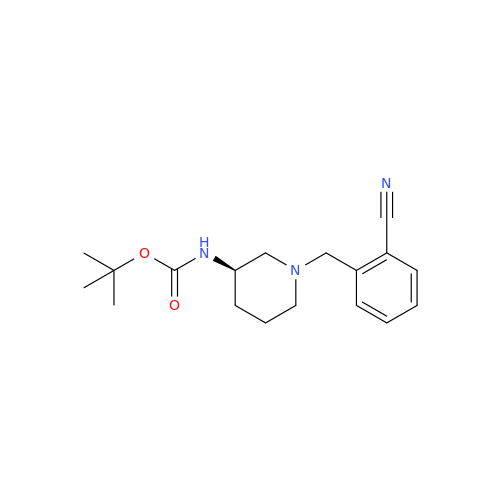
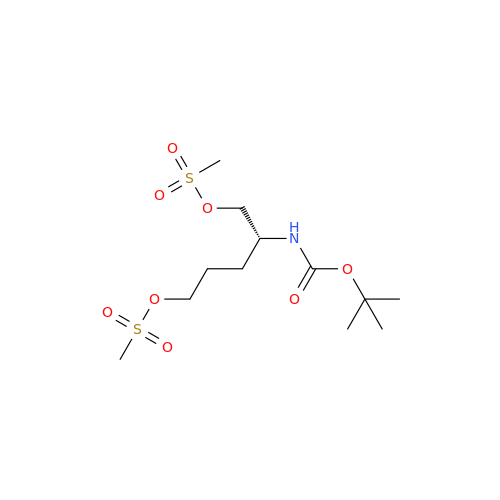
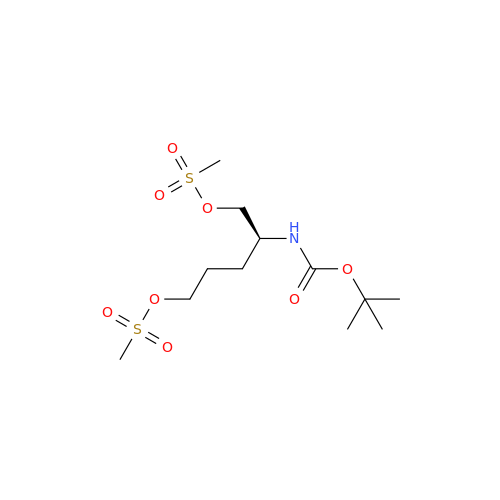
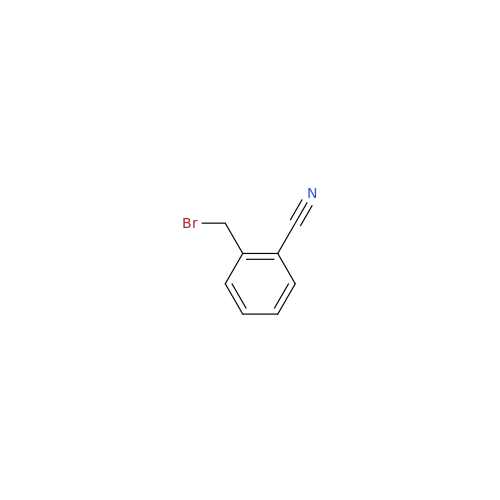
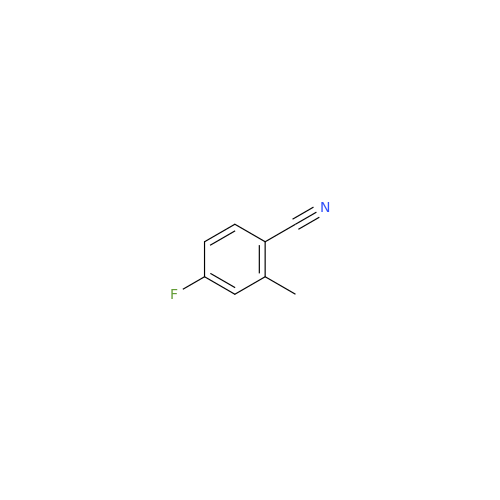
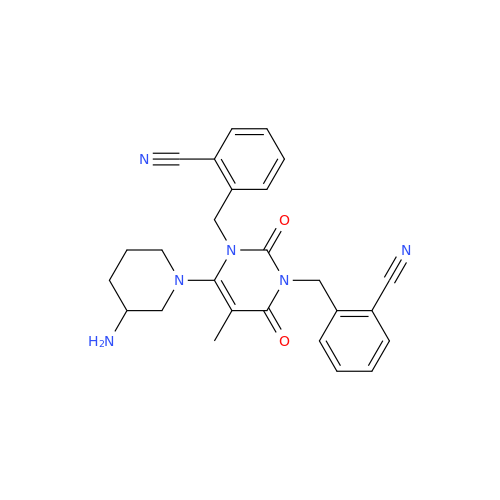
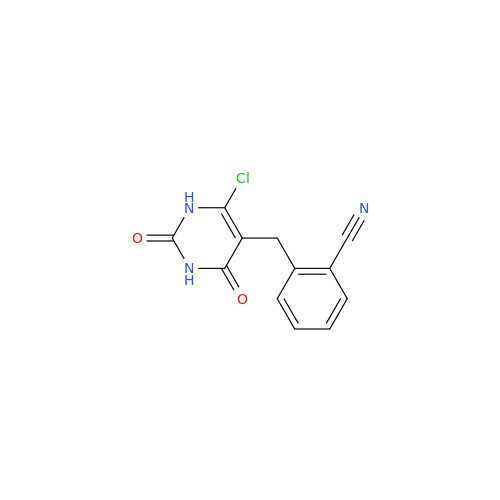
Alpelisib Impurity 2
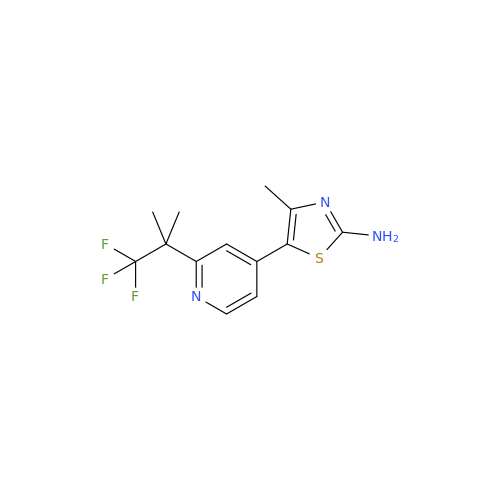
|
Chemical Name: Alpelisib Impurity 2
Synonym: 4-Methyl-5-(2-(1,1,1-trifluoro-2-methylpropan-2-yl)pyridin-4-yl)thiazol-2-amine| Enter Batch Number | |||