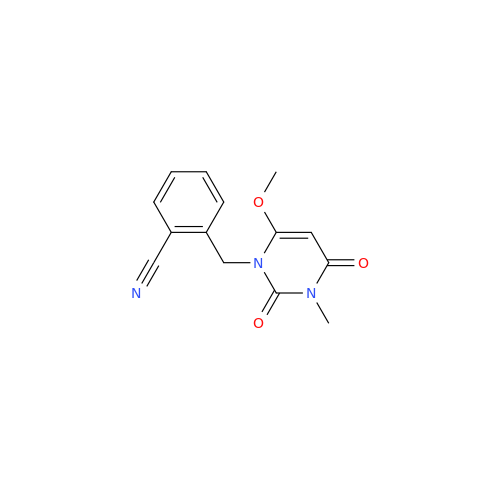
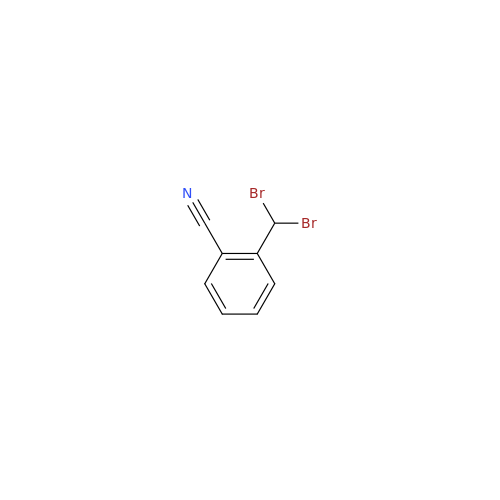
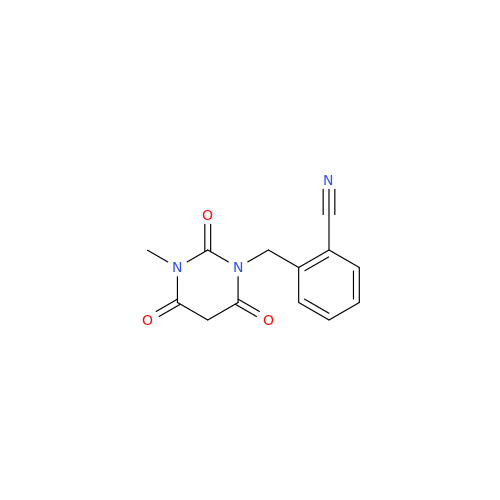
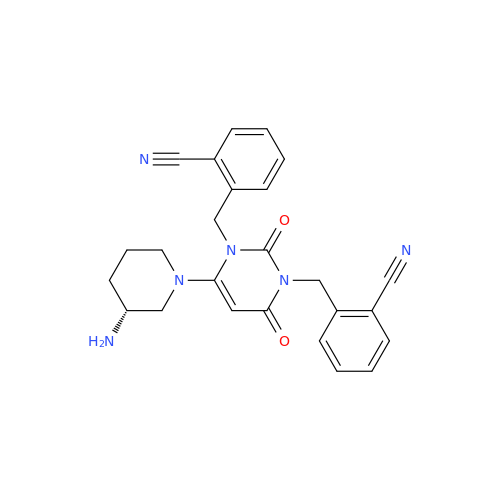
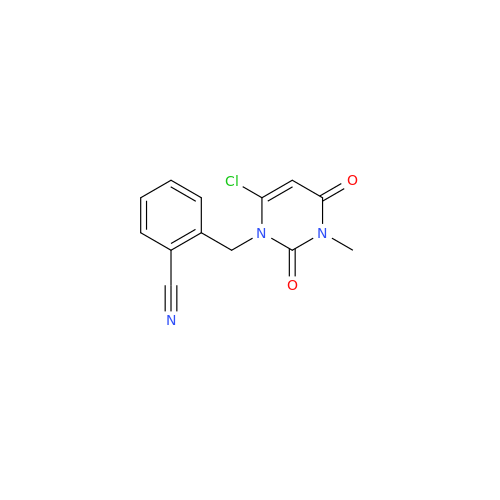
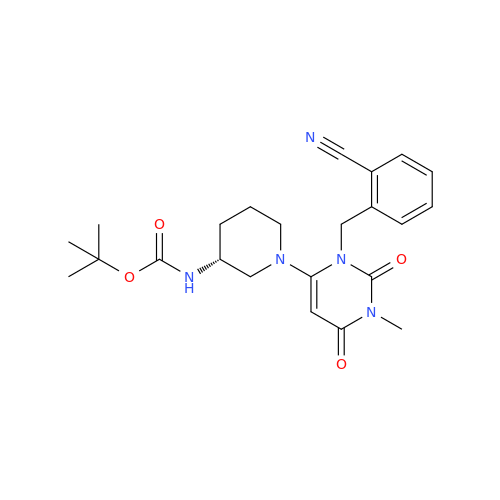
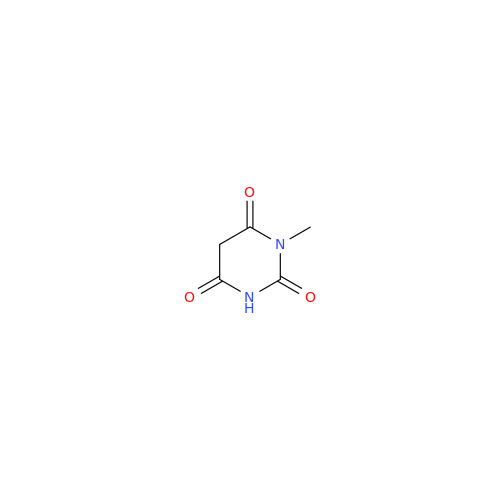
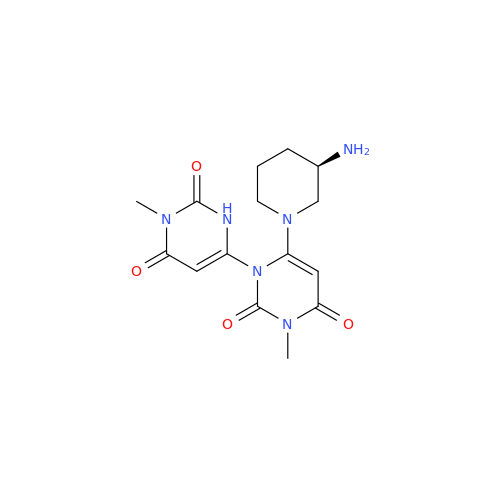
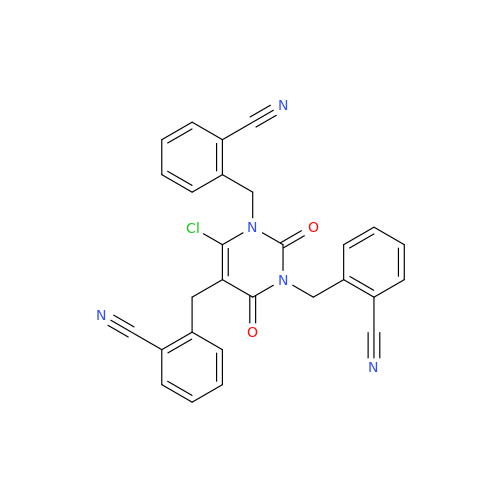
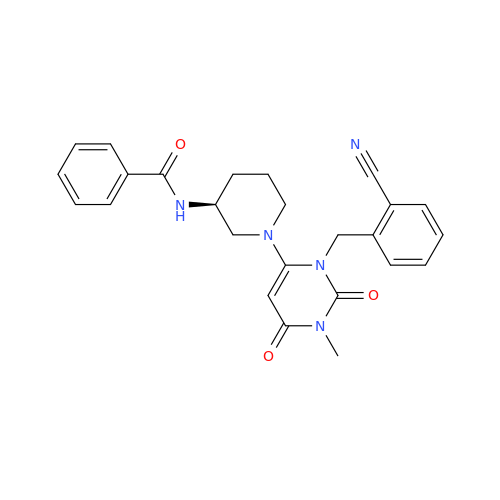
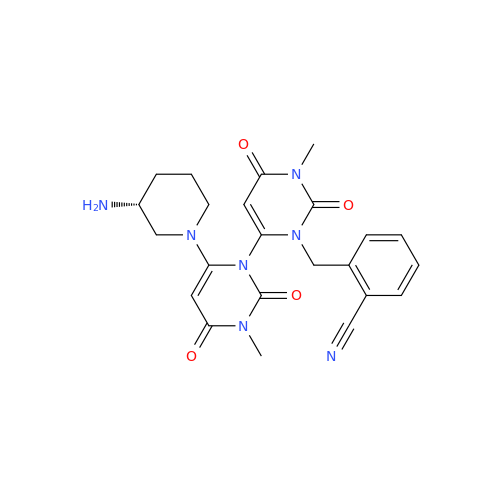
Product Information
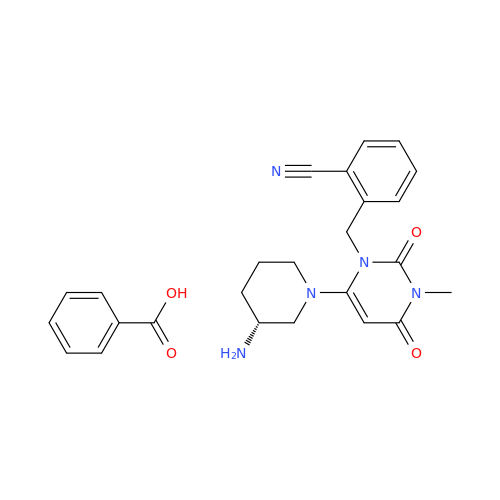
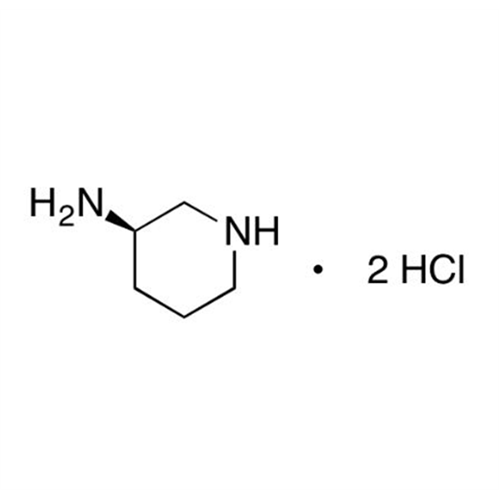
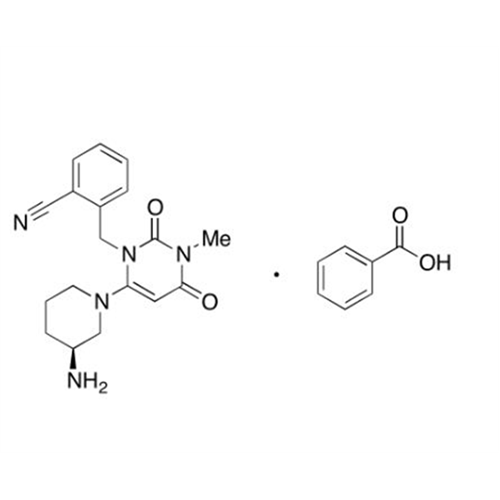
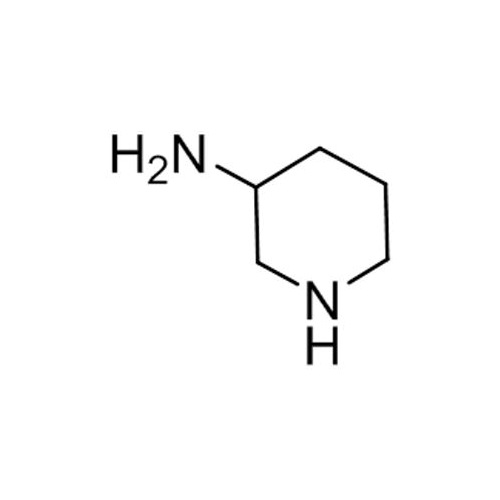
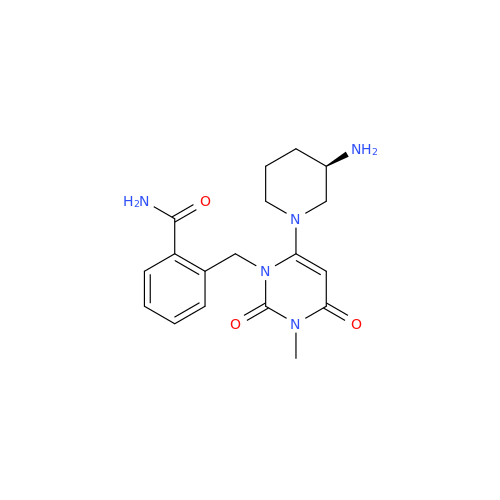
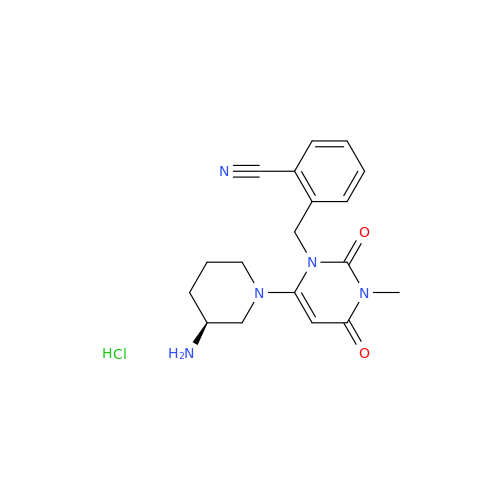
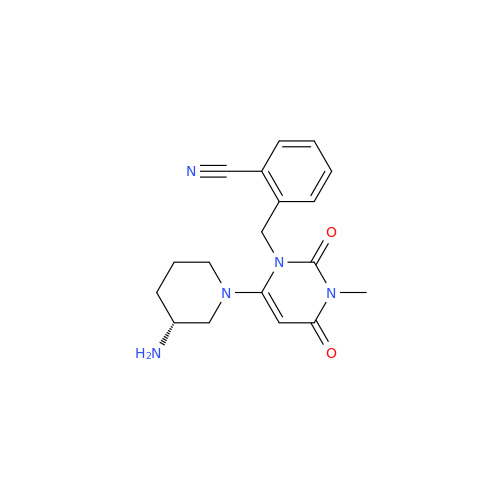
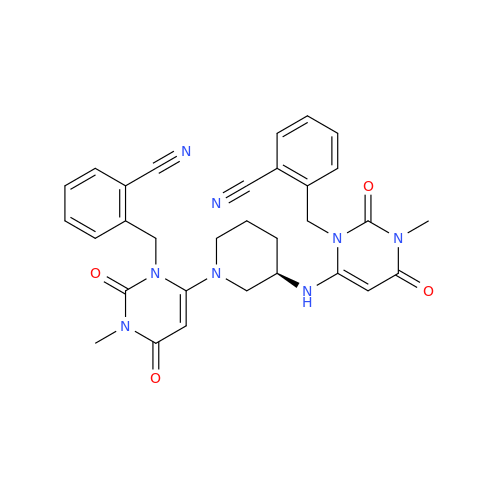
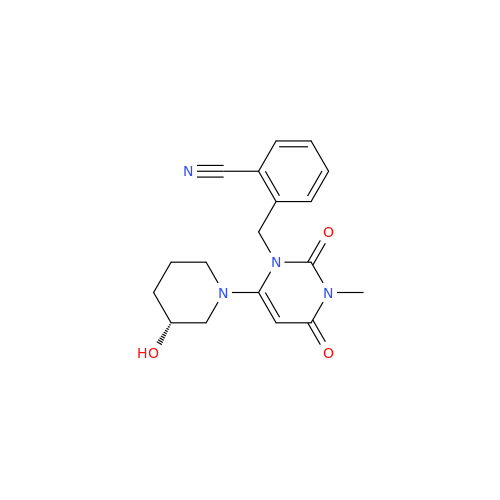
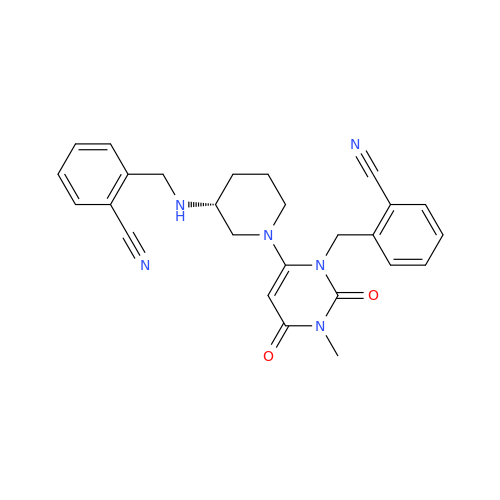
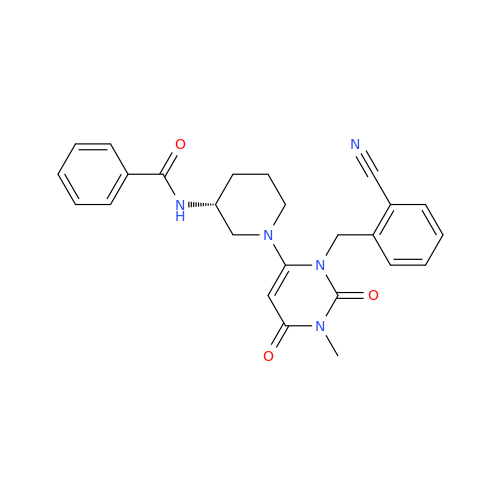
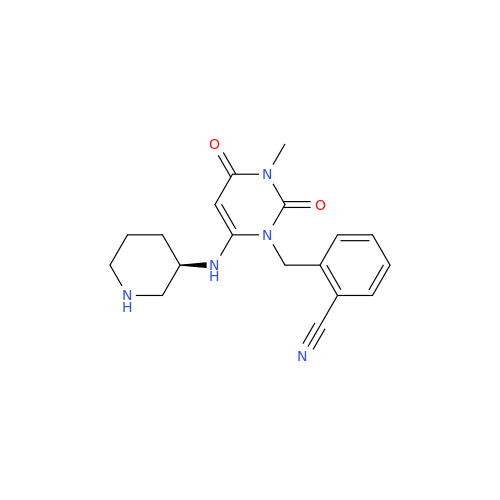
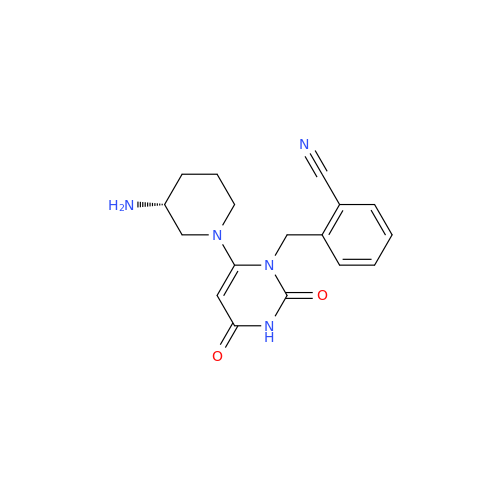
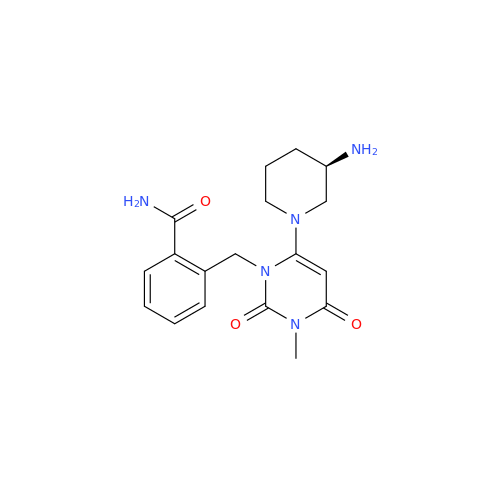
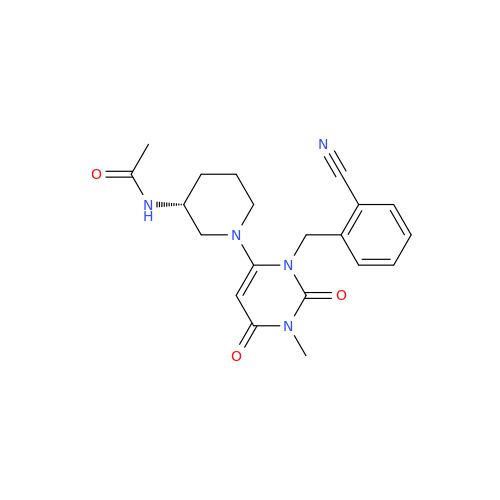
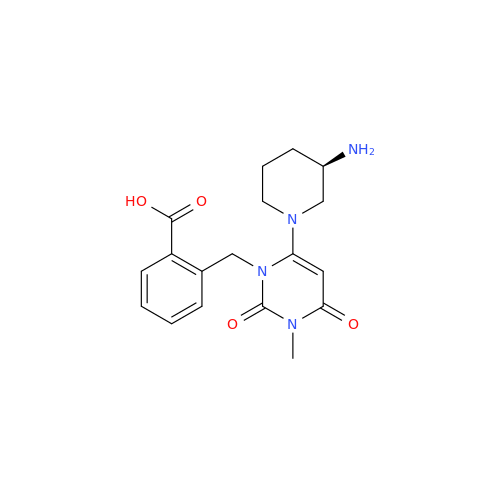
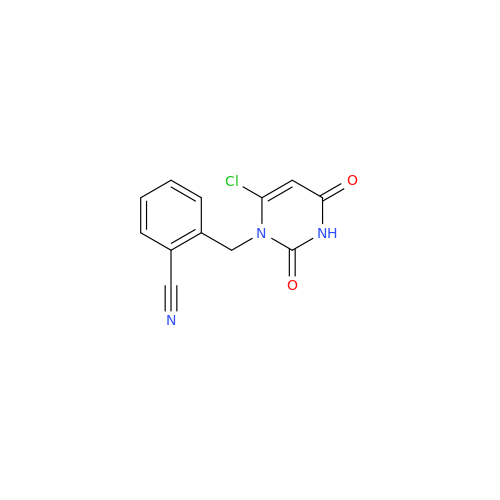
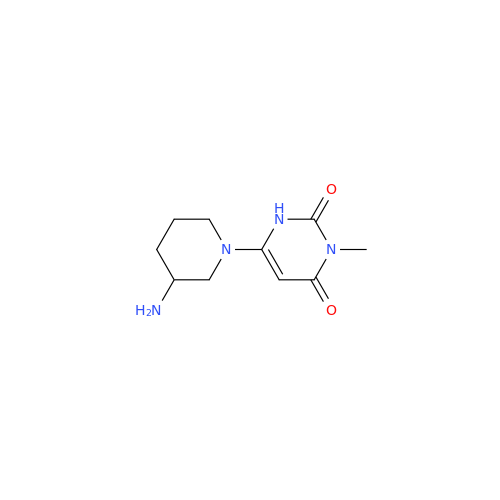
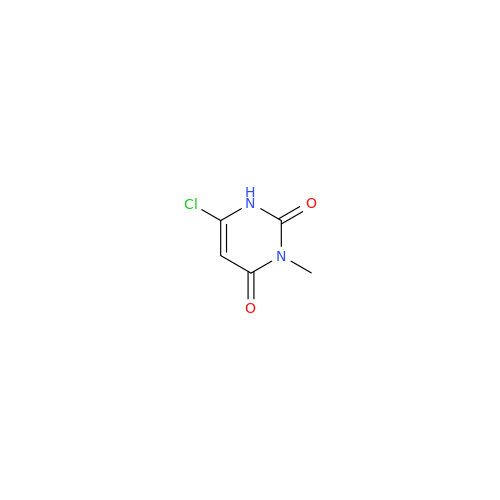
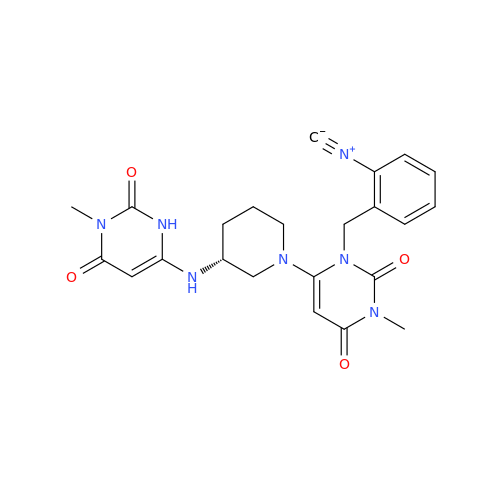
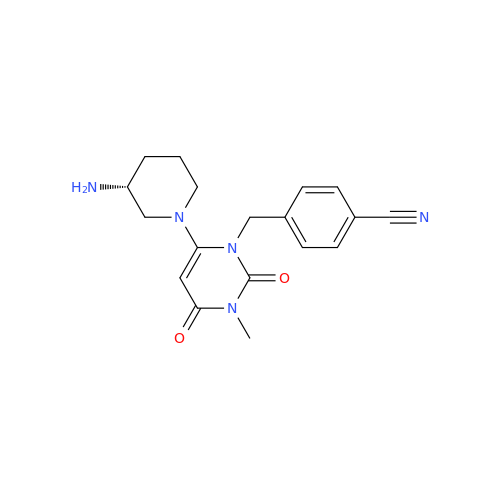
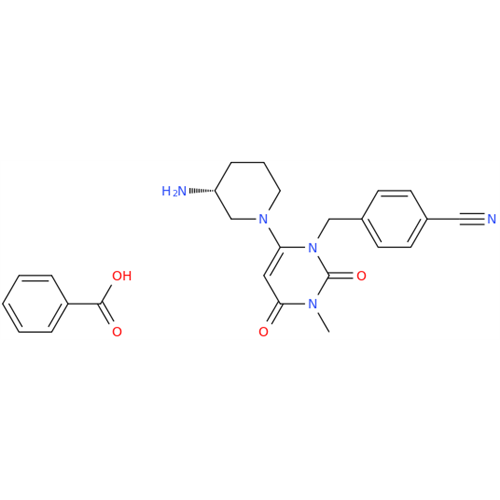
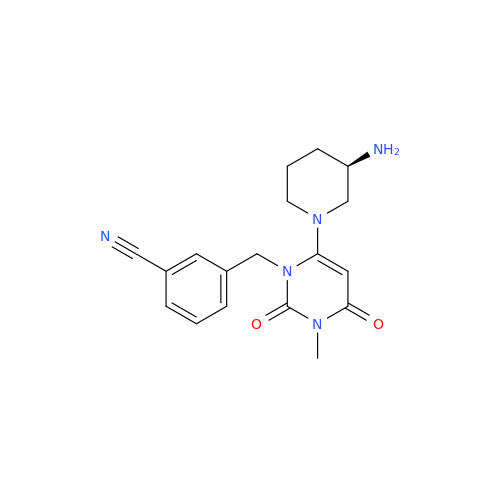
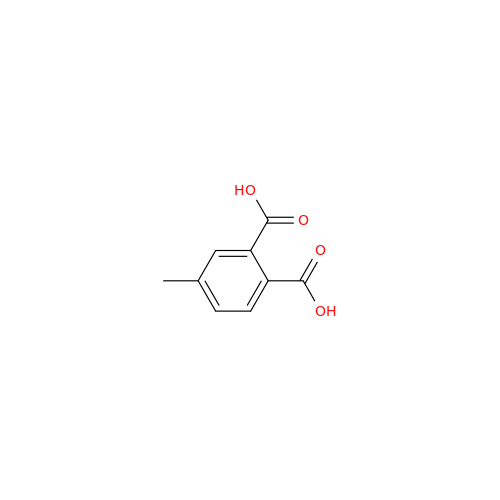
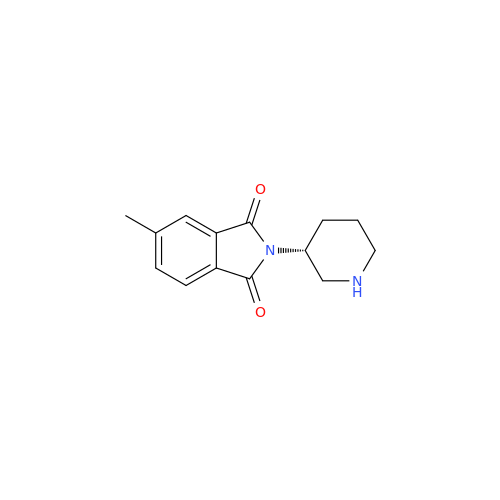
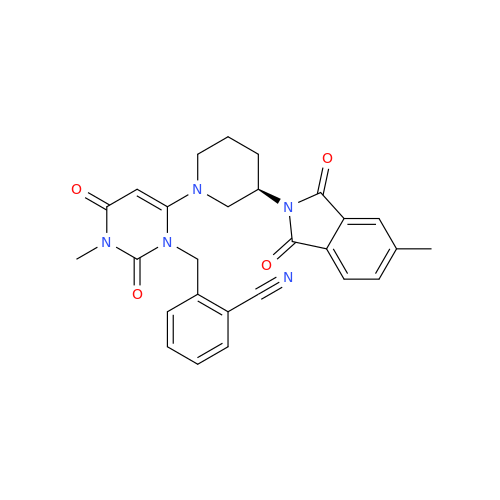
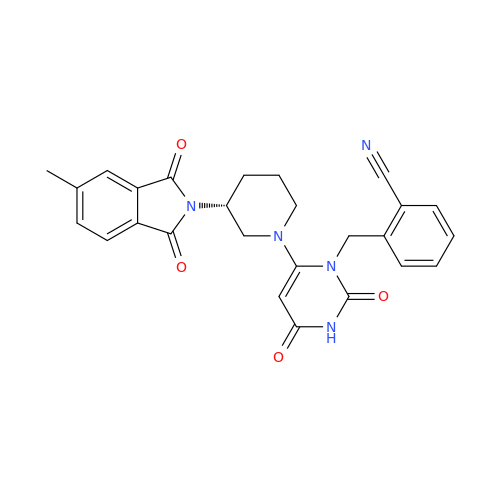
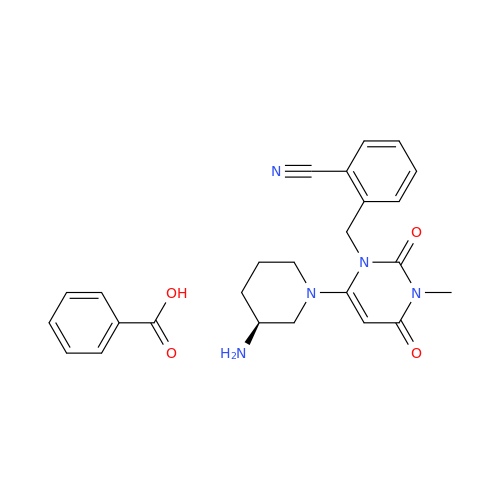
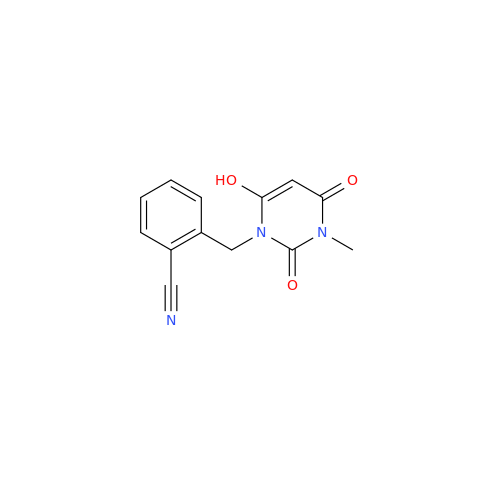
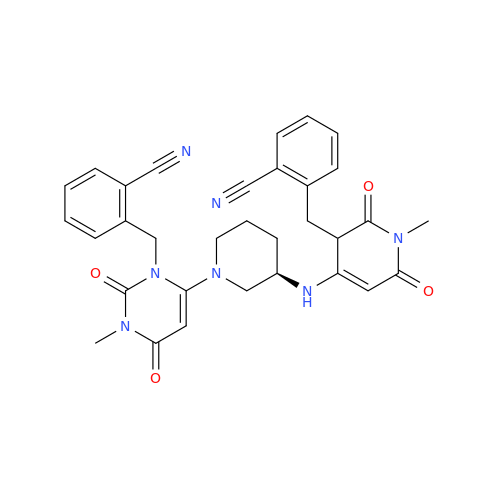
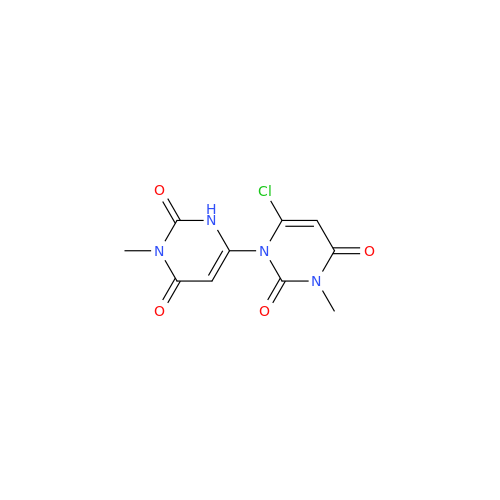
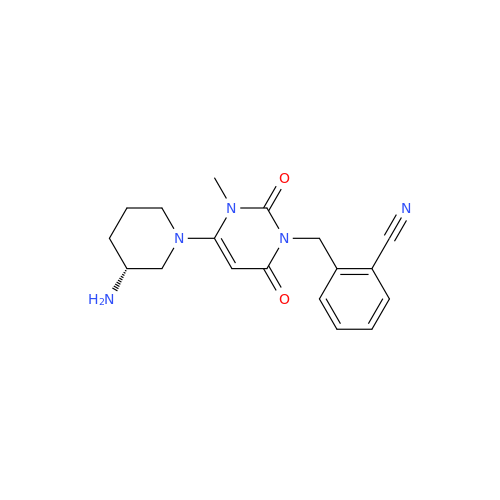
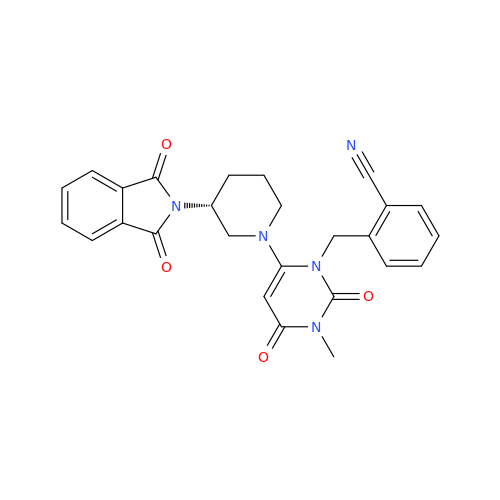
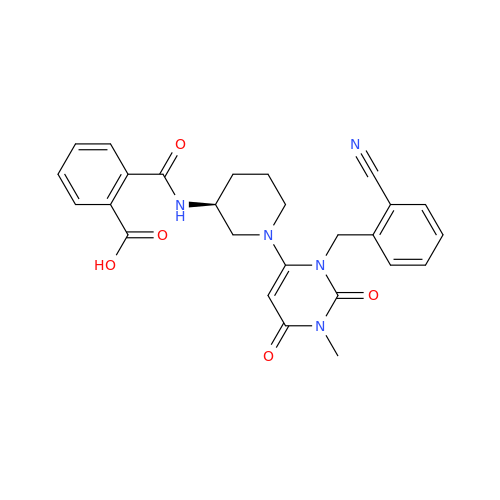
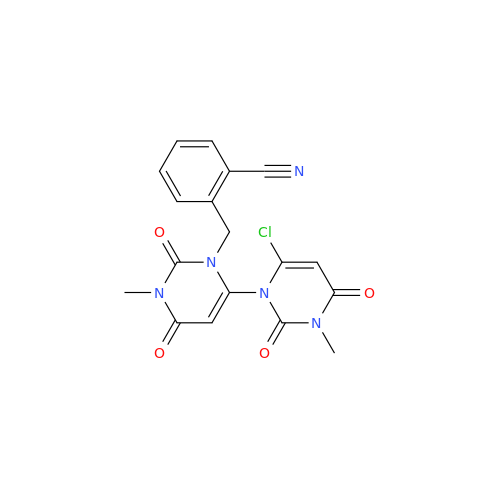
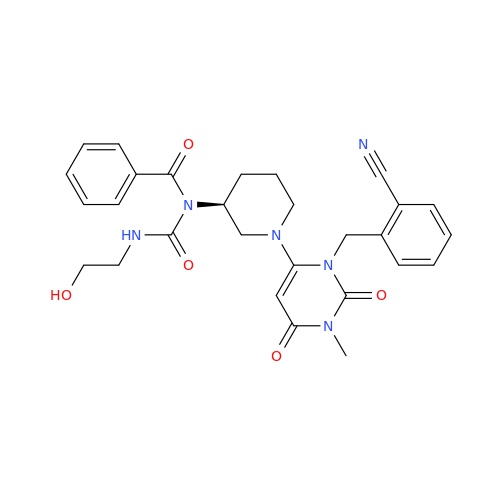
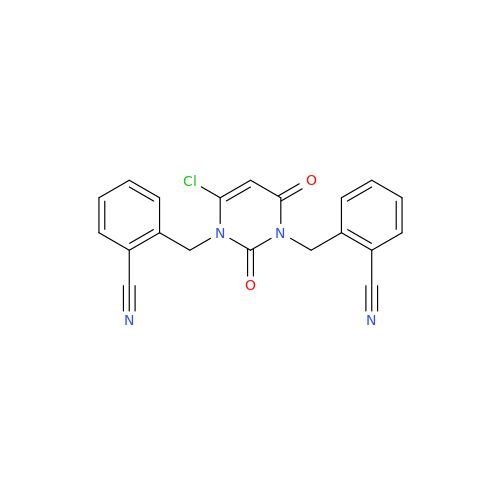
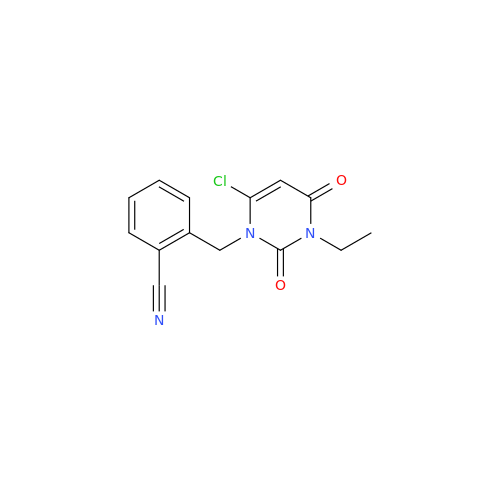
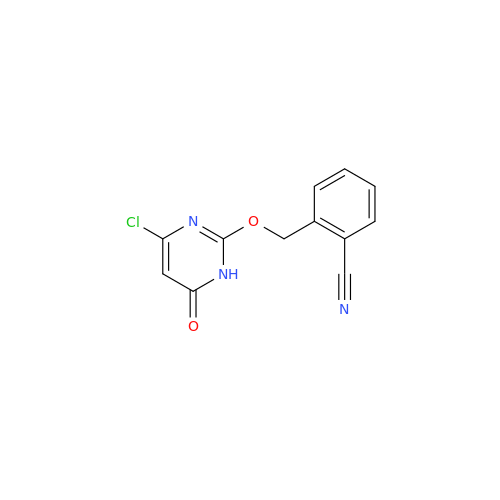
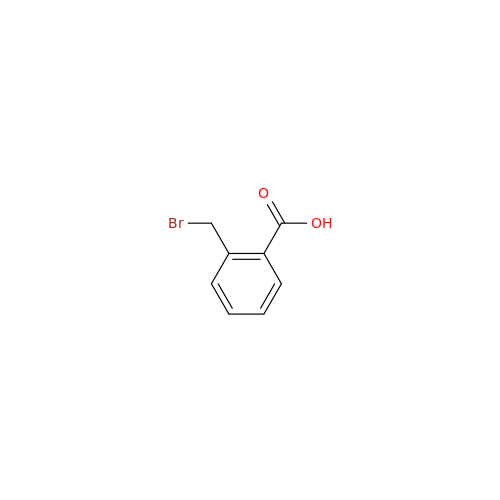
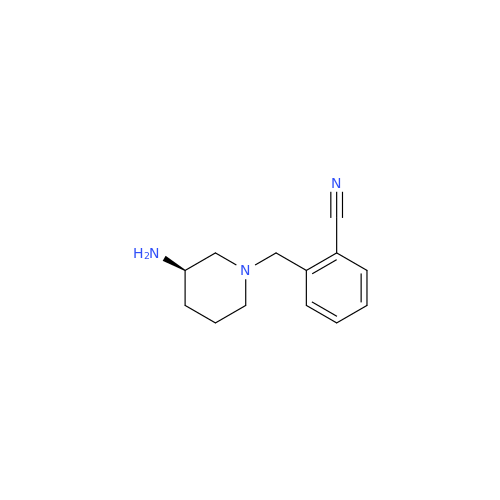
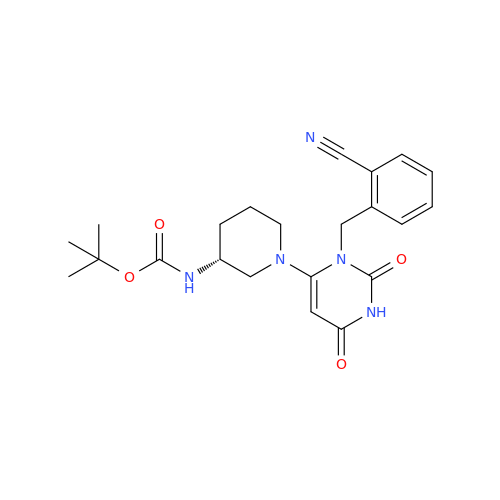
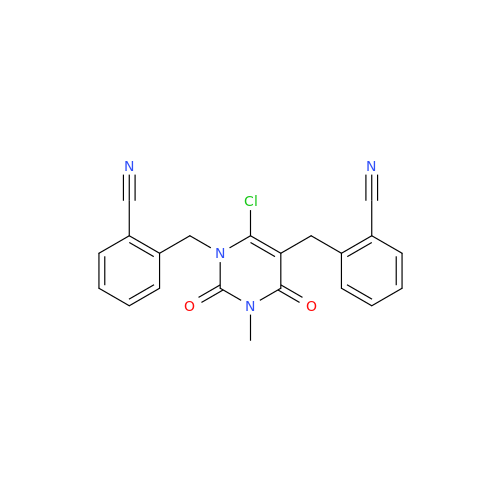
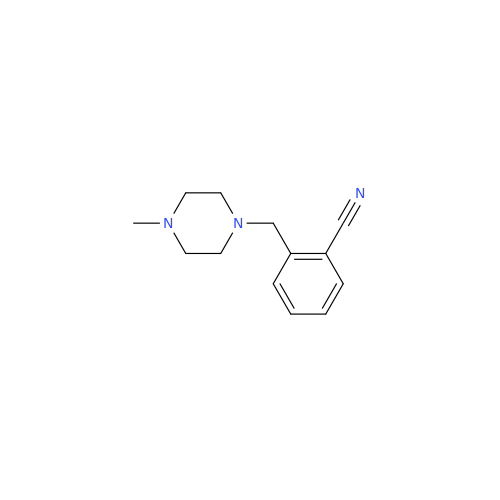
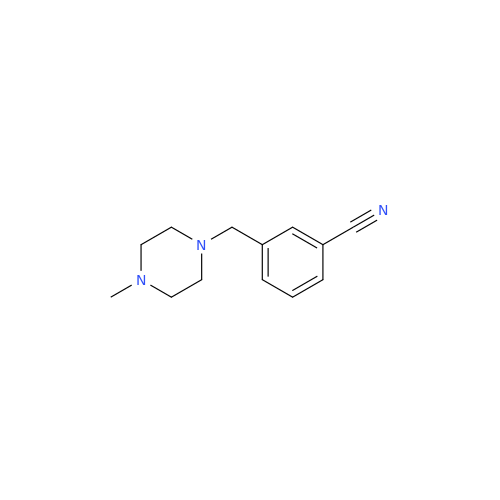
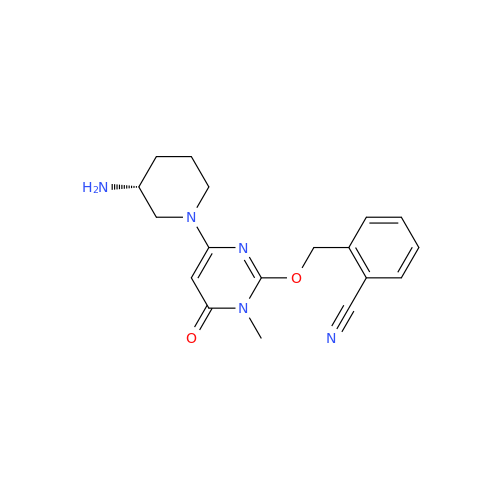
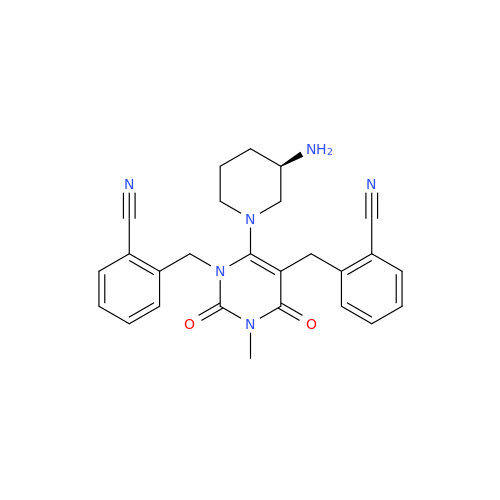
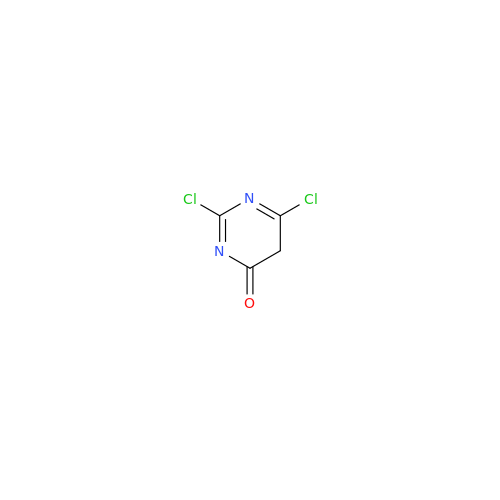
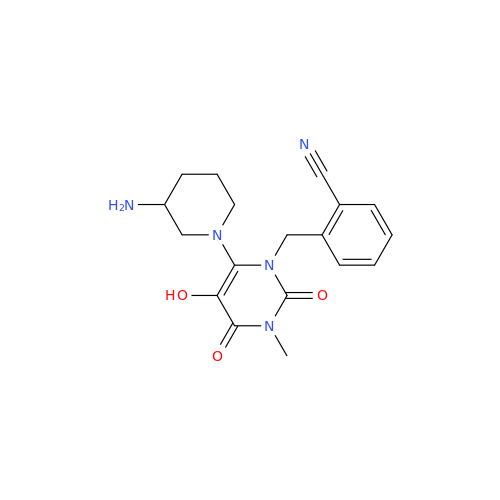
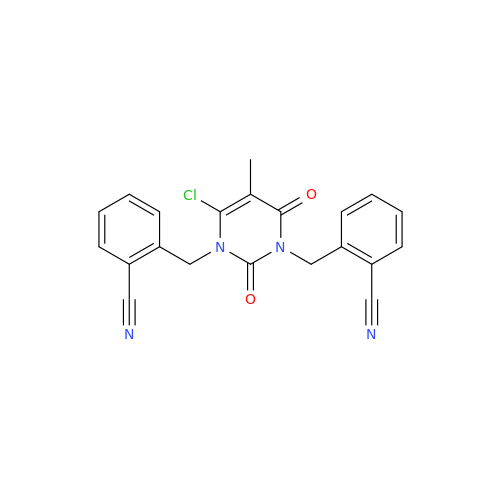
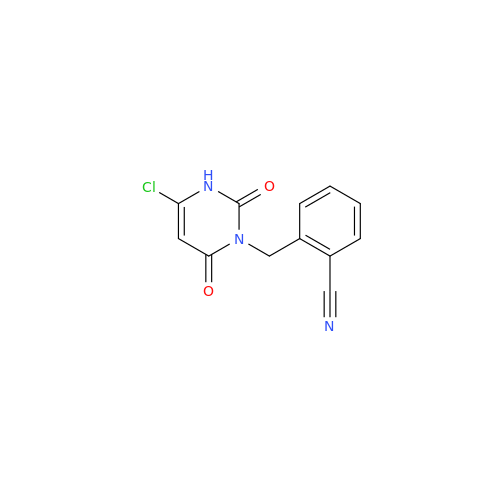
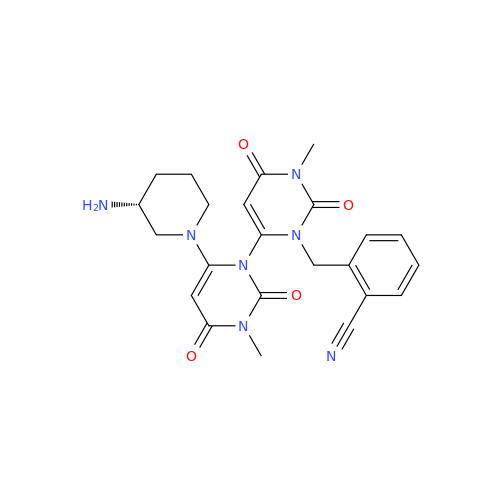
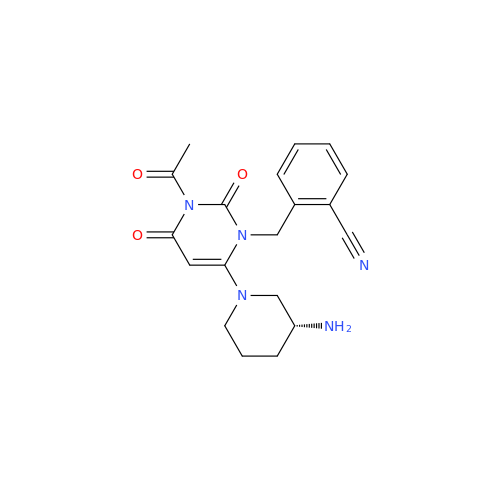
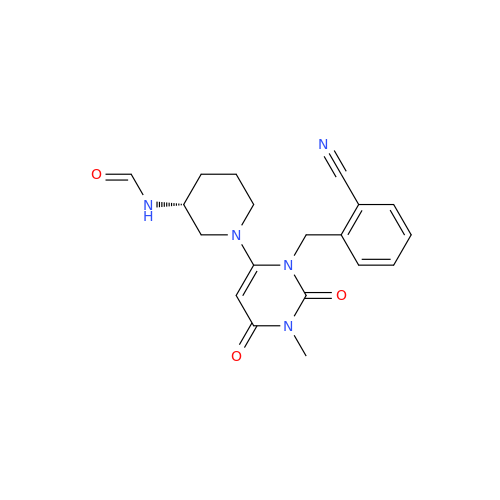
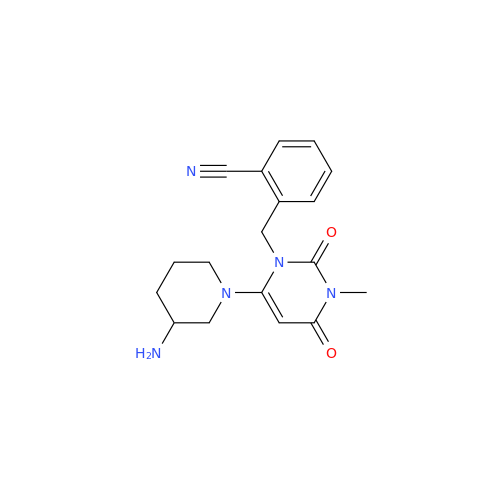
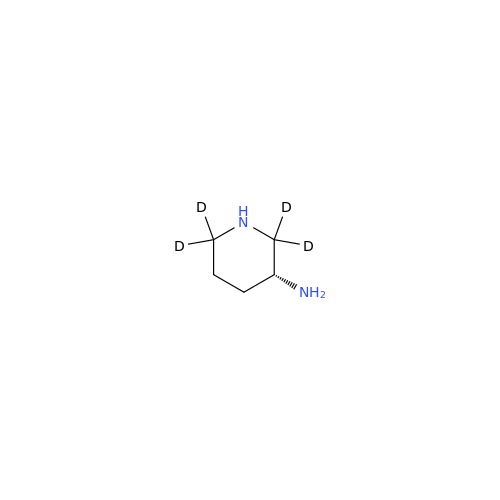
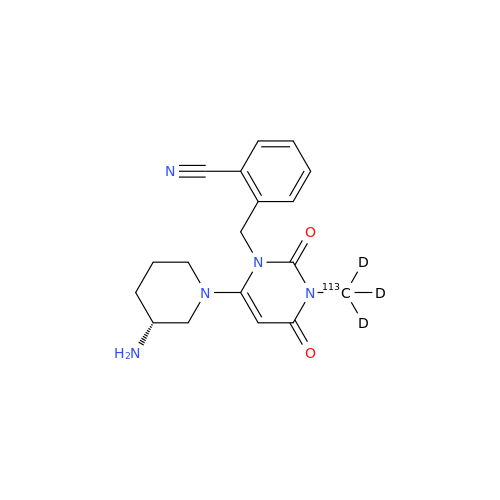
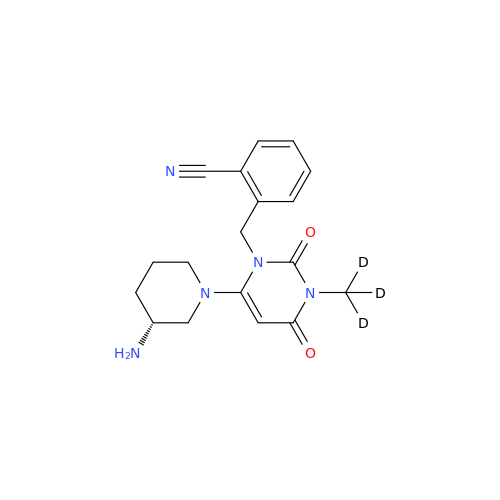
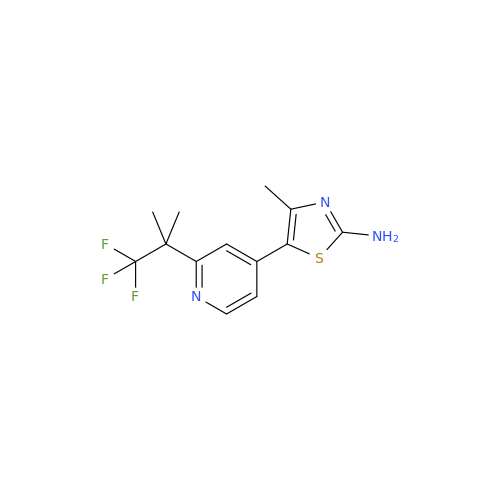
Alogliptin Impurity 64
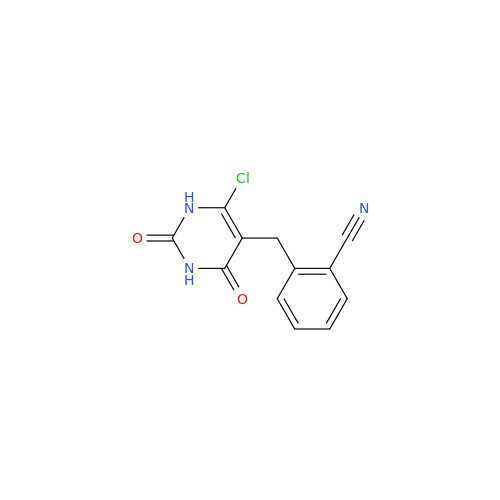
|
Chemical Name: Alogliptin Impurity 64
Synonym: 2-((6-Chloro-2,4-dioxo-1,2,3,4-tetrahydropyrimidin-5-yl)methyl)benzonitrile| Enter Batch Number | |||