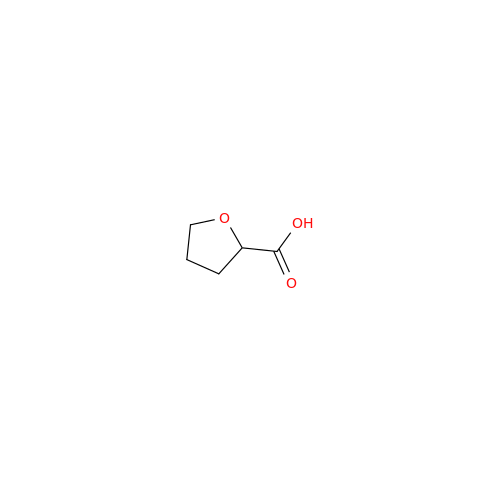
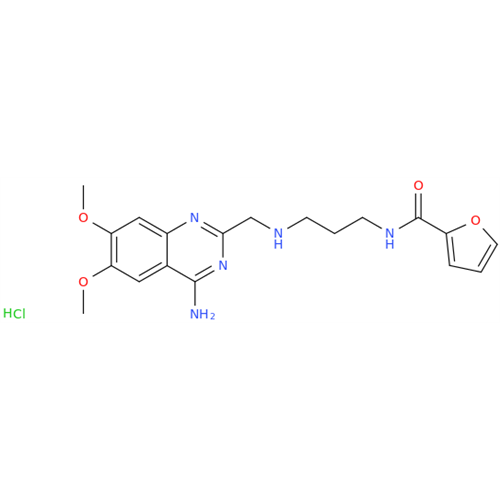
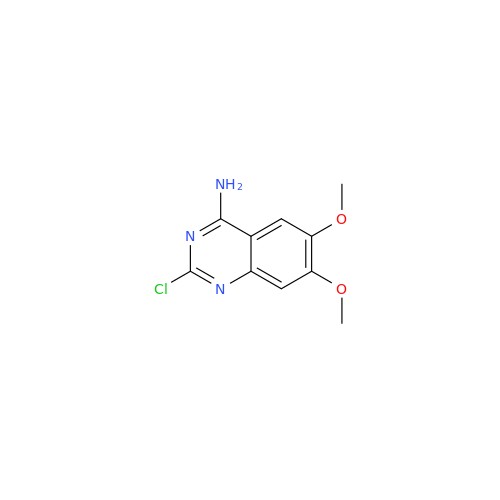
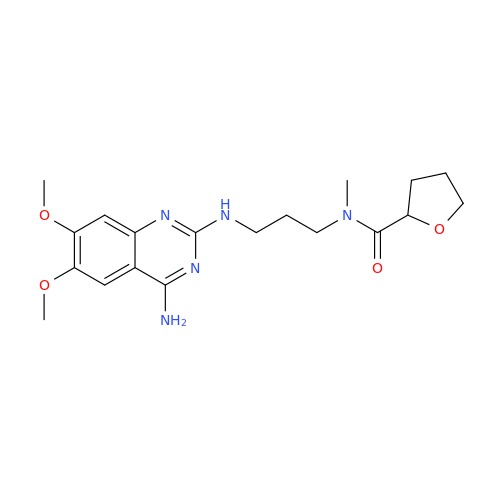
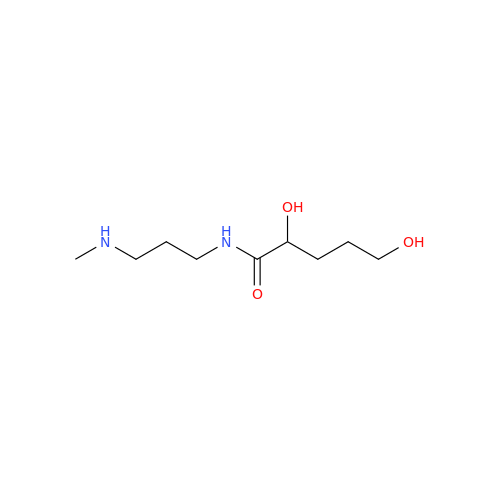
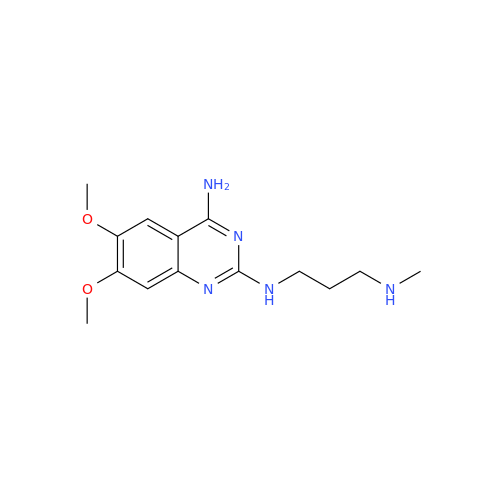
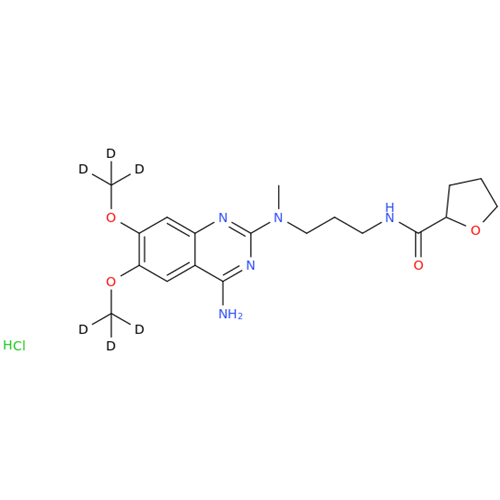
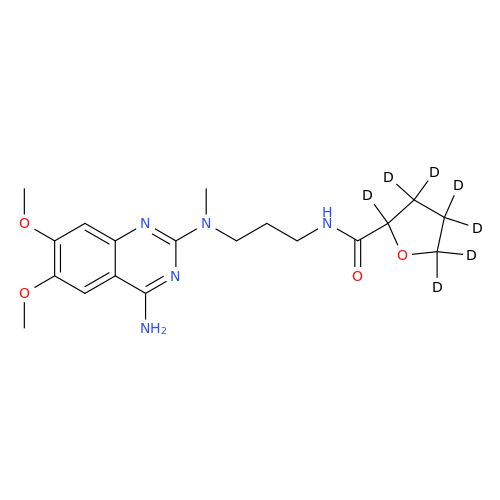
Product Information
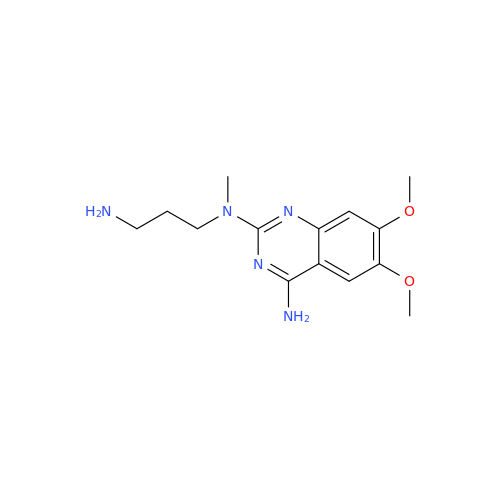
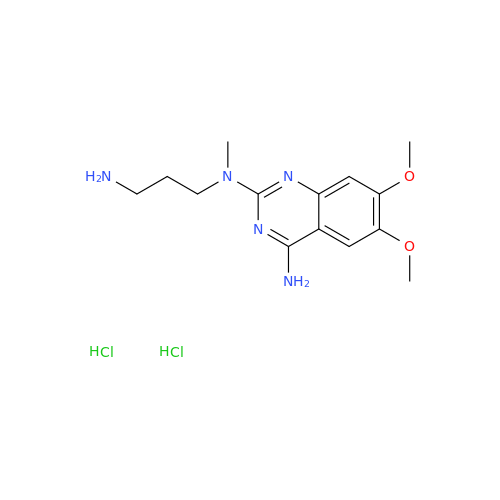
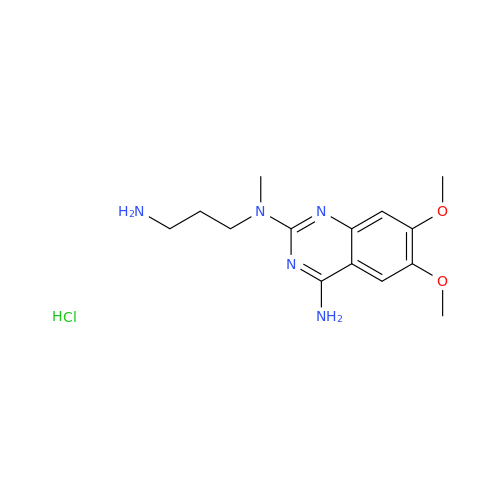
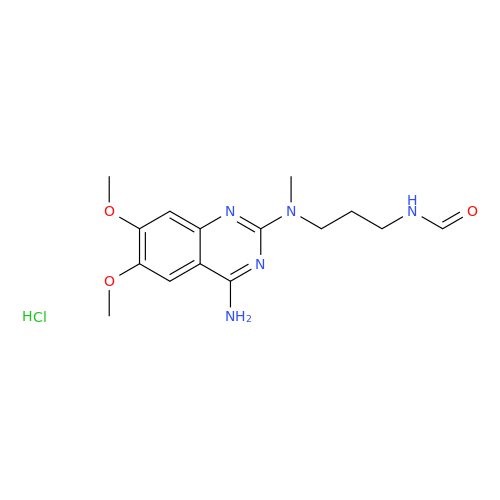
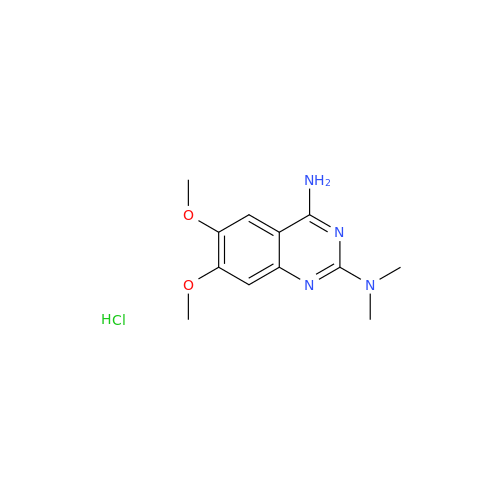
Alfuzosin EP Impurity C
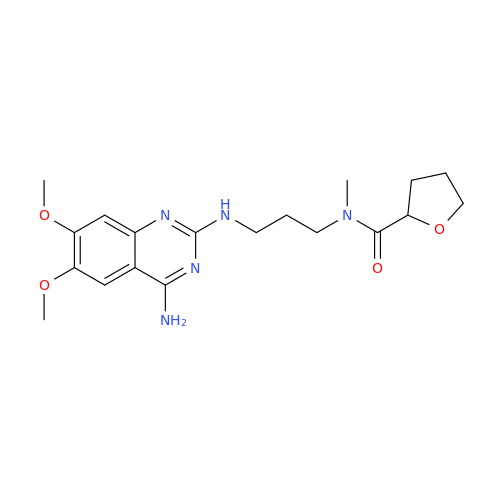
|
Chemical Name: Alfuzosin EP Impurity C
Synonym: 2-Chloro-6,7-dimethoxy-4-quinazolinamine| Enter Batch Number | |||