Product Information
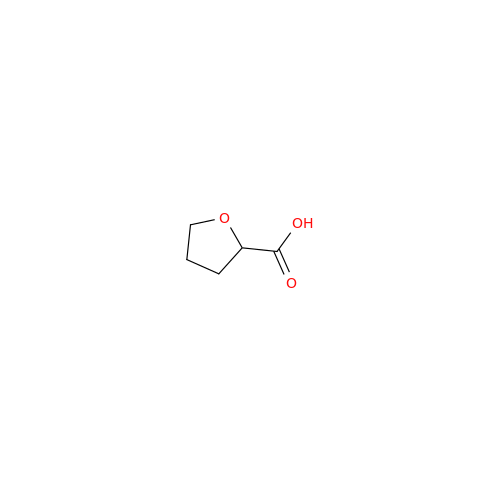
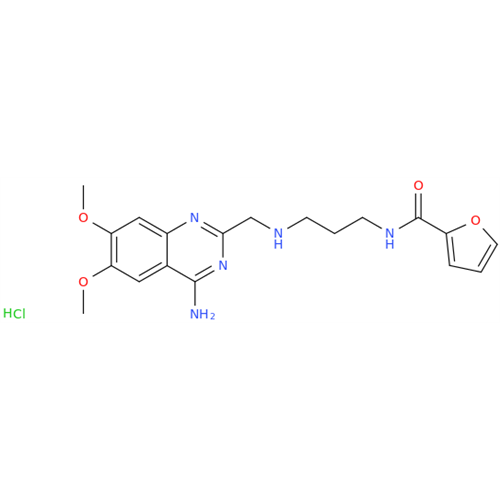
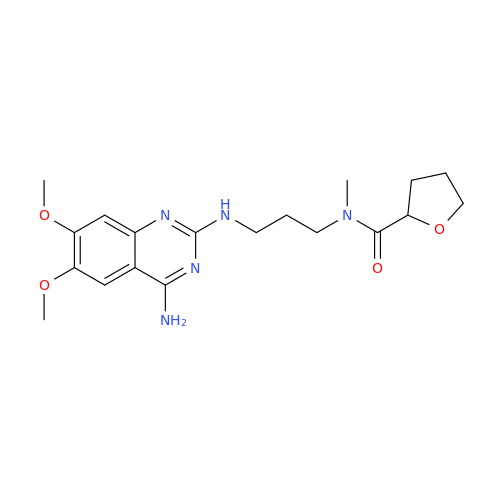
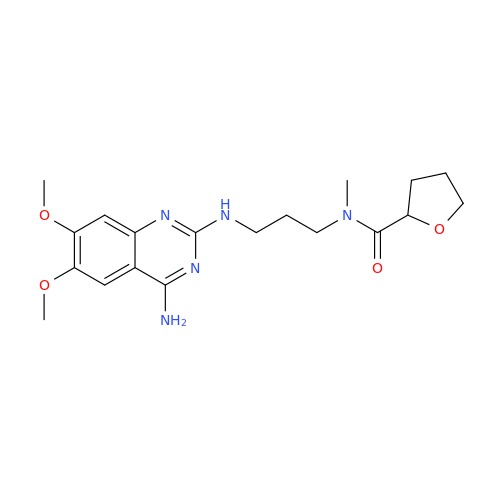
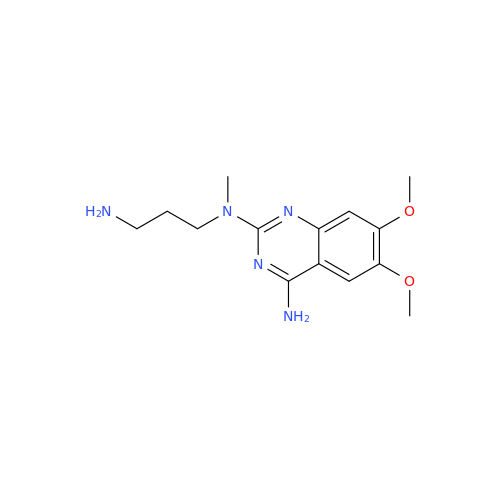
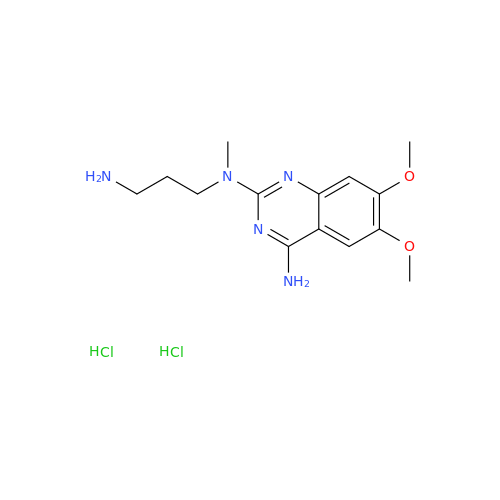
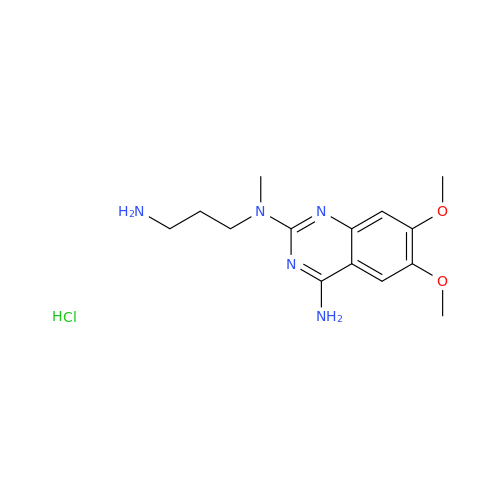
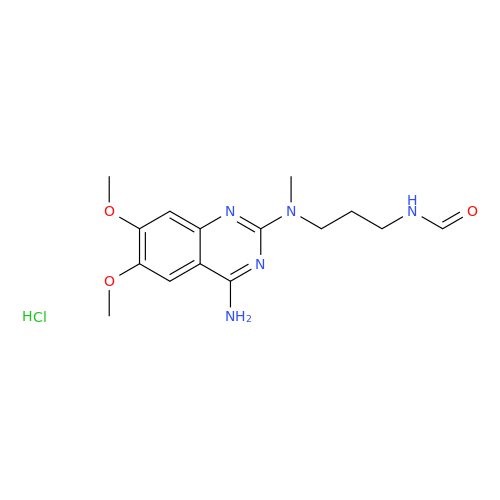
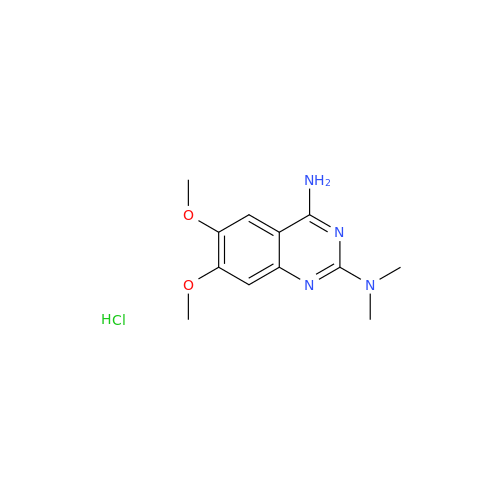
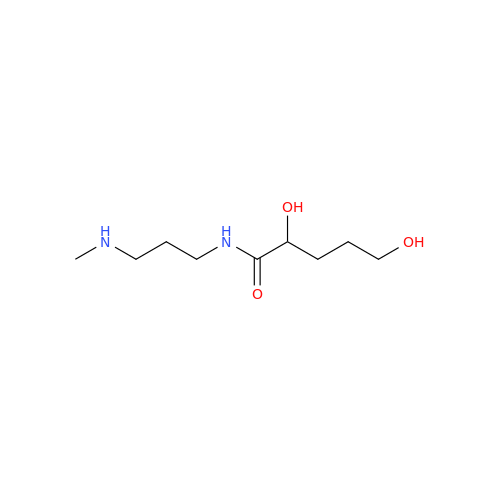
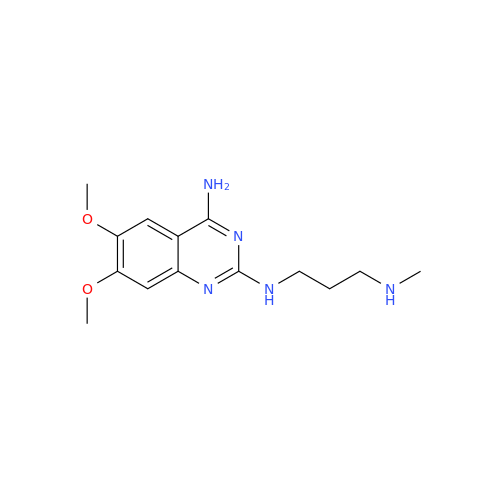
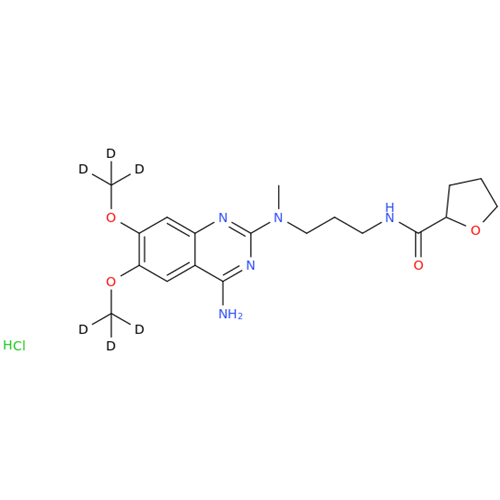
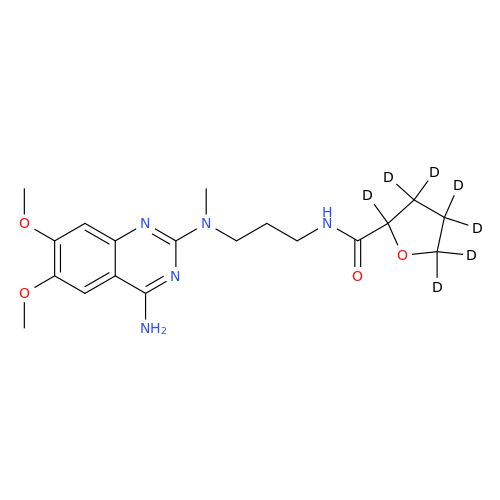
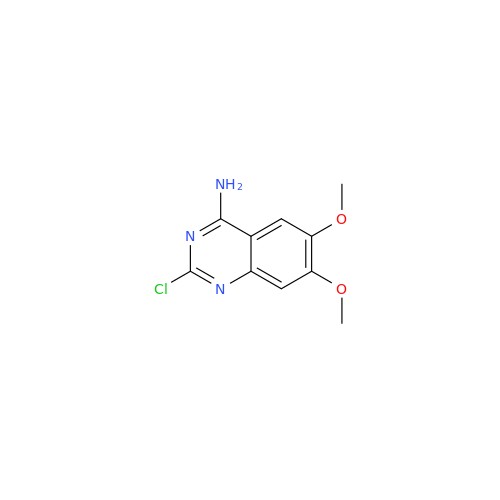
Alfuzosin EP Impurity B
|
Chemical Name: Alfuzosin EP Impurity B
Synonym: 2-Chloro-6,7-dimethoxy-4-quinazolinamine| Enter Batch Number | |||