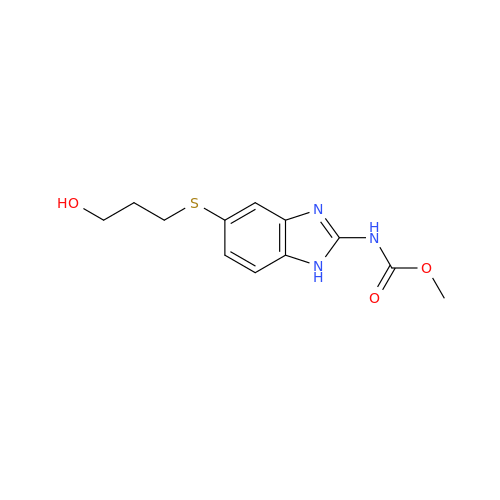
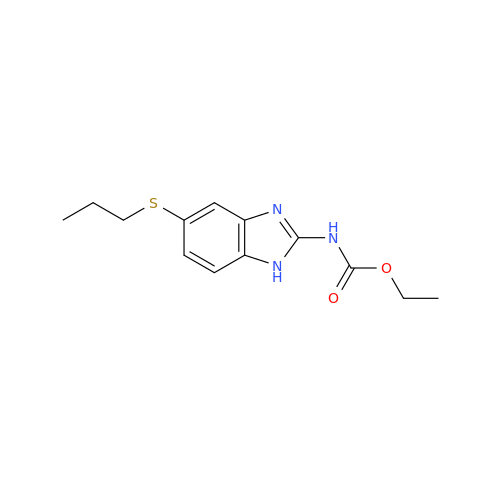
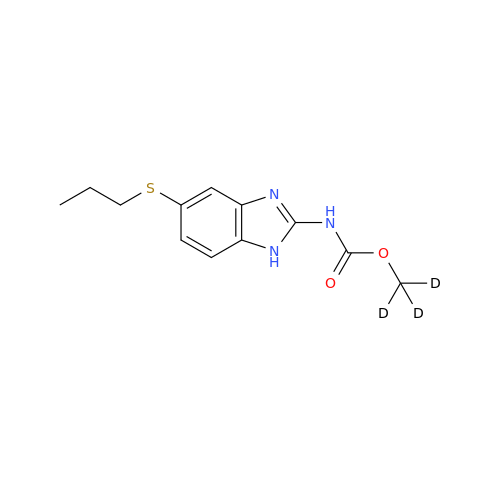
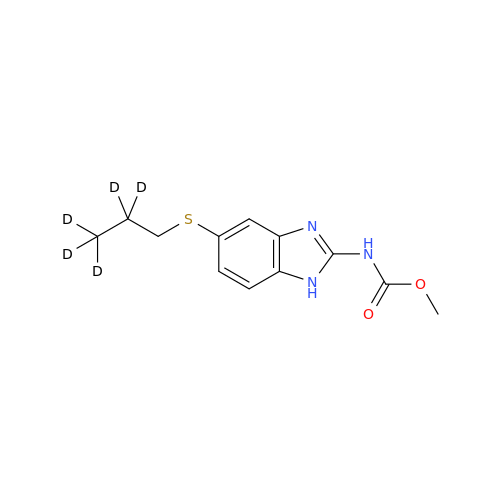
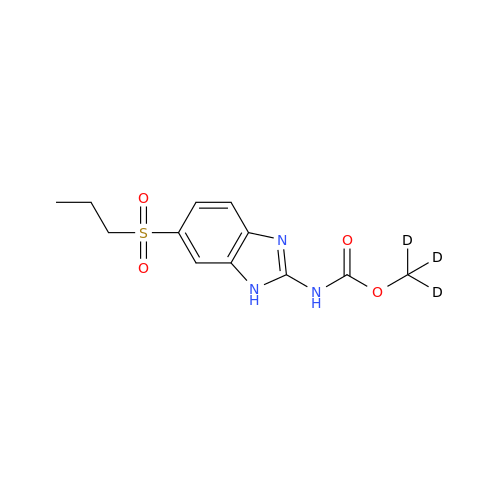
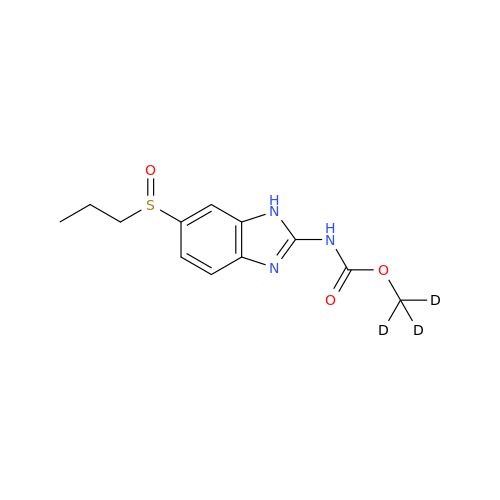
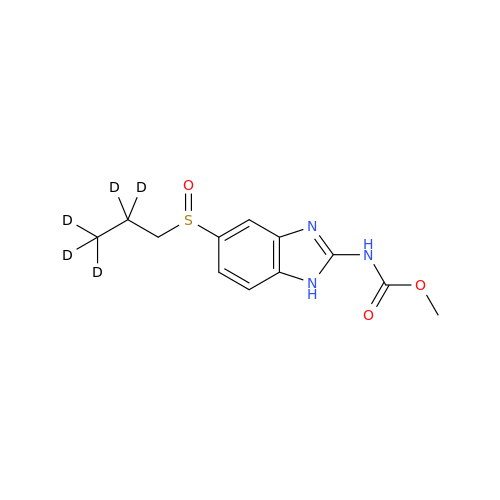
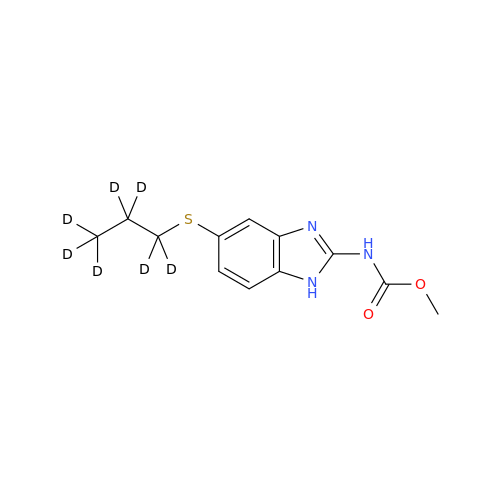
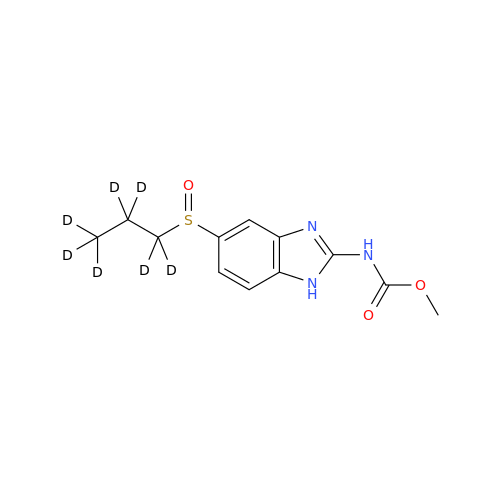
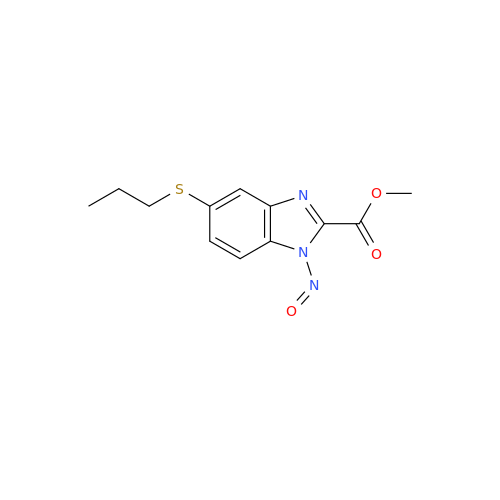
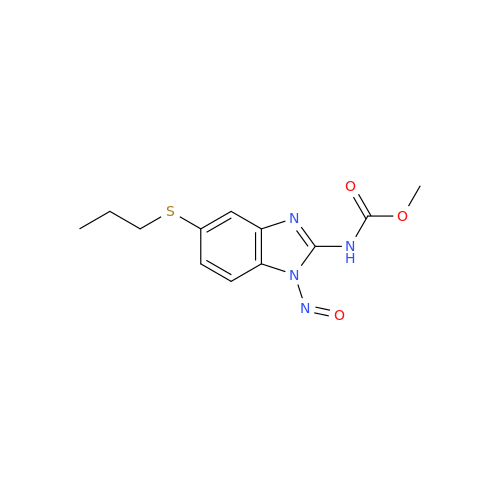
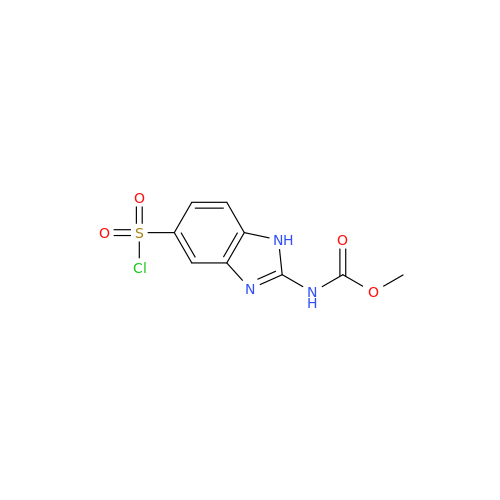
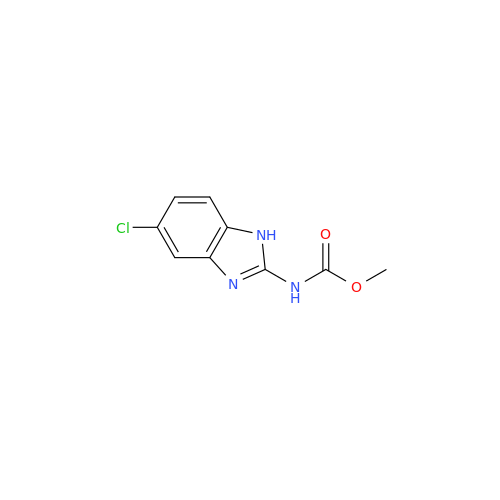
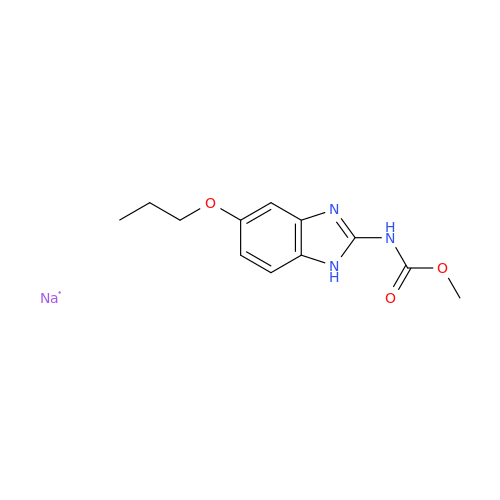
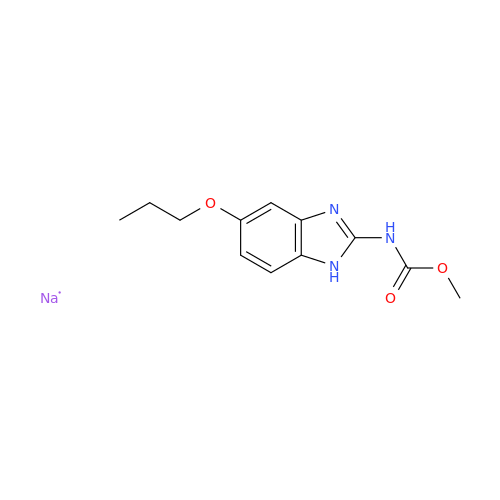
Product Information
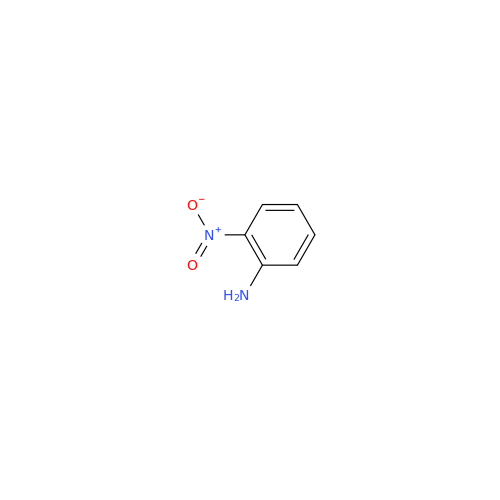
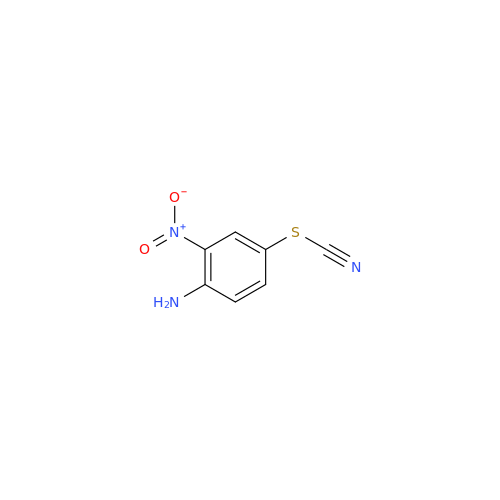
2-Nitro-4-(propylthio)aniline
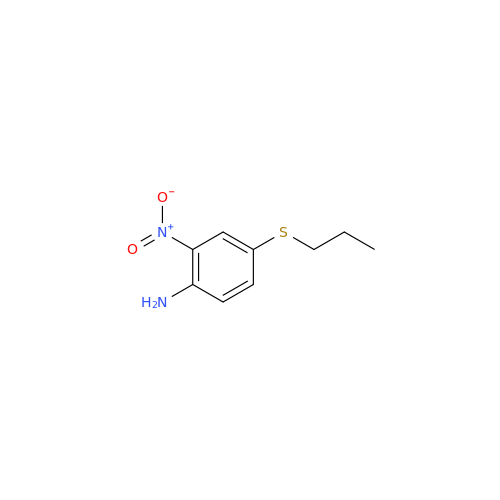
|
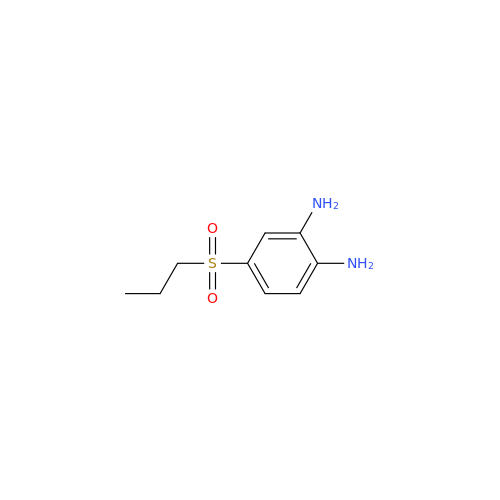
Chemical Name: 2-Nitro-4-(propylthio)aniline
Synonym: 2-Nitro-4-(propylthio)benzenamine; Albendazole Impurity 3| Enter Batch Number | |||



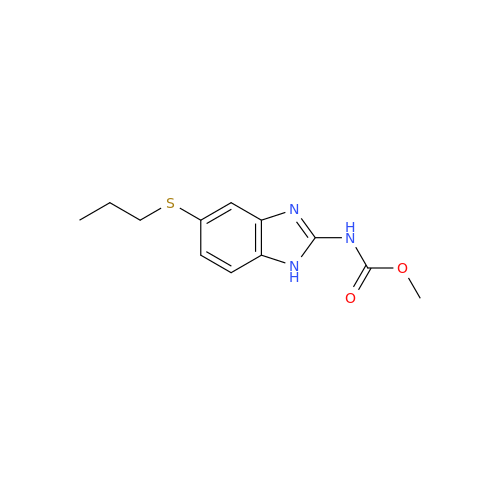
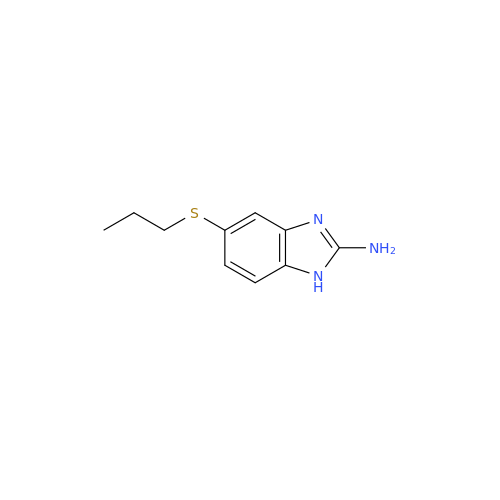
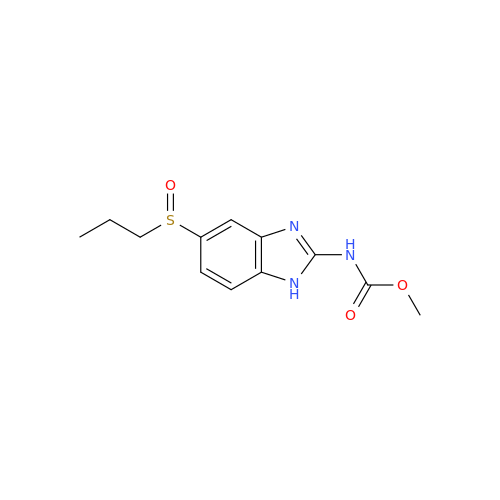
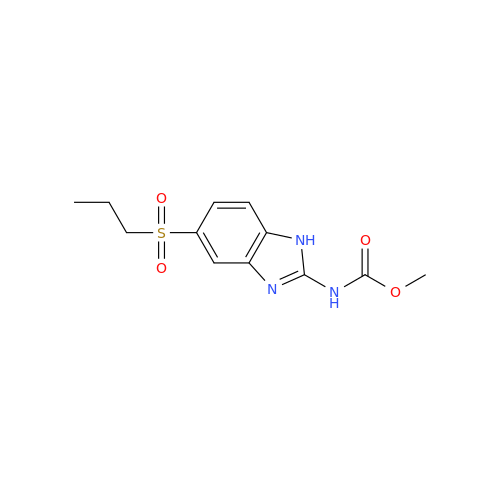
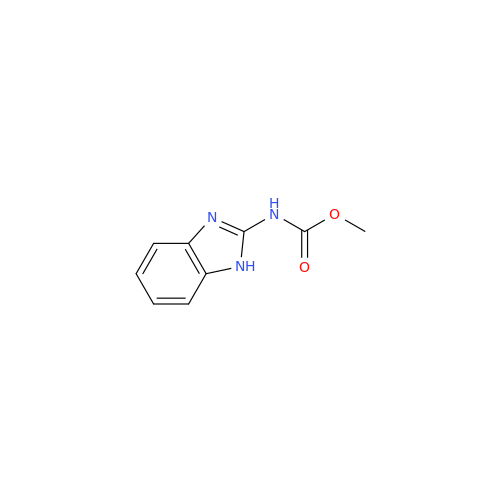
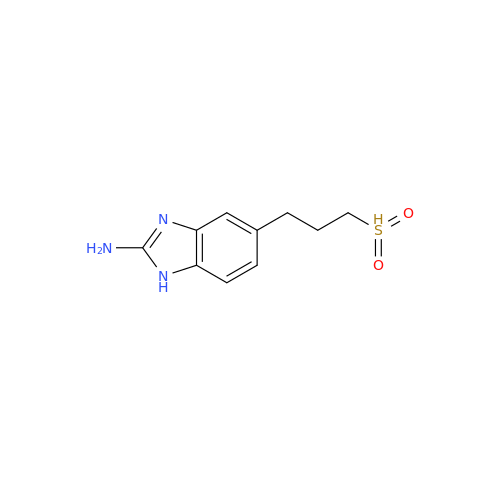
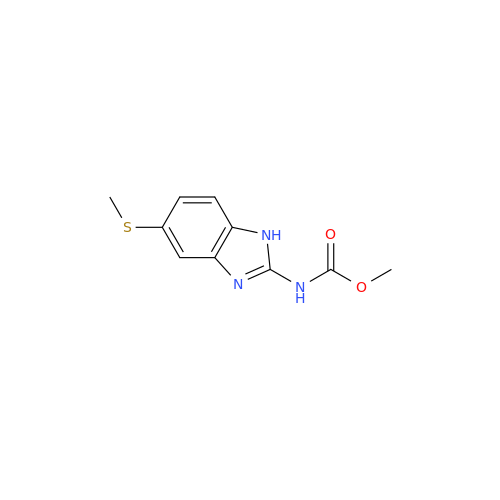
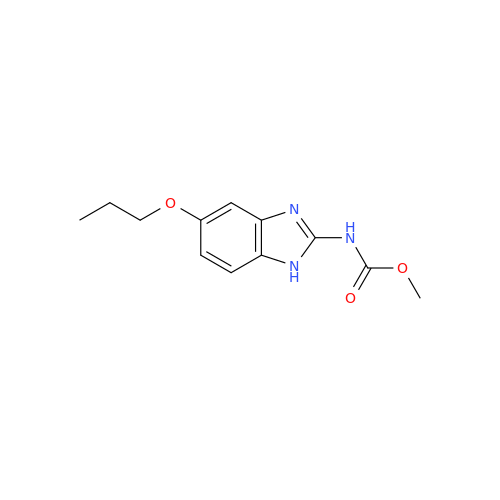
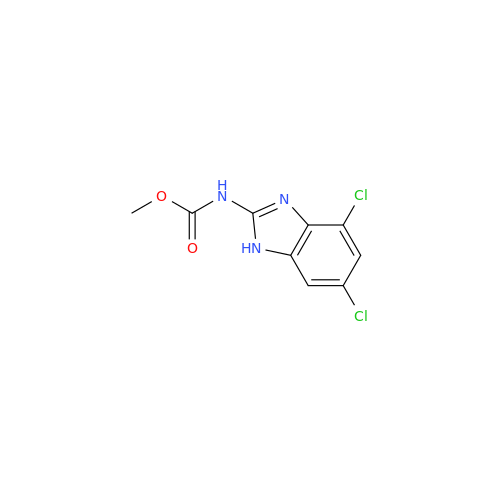
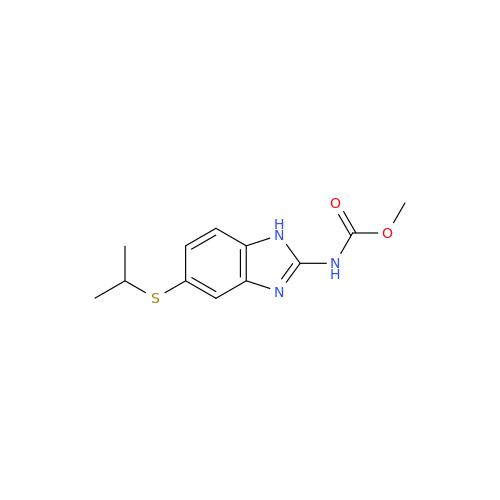
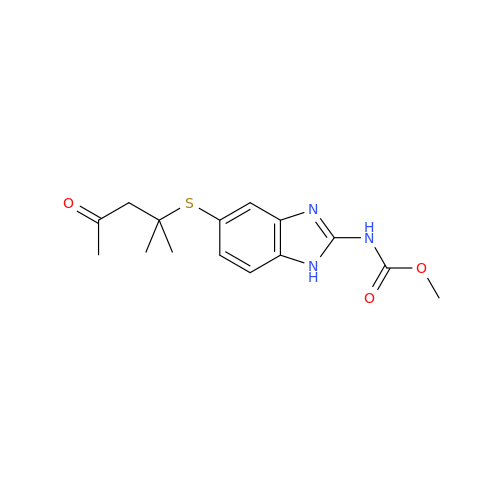
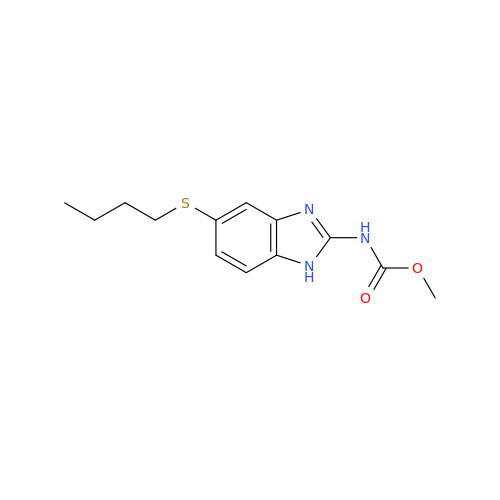
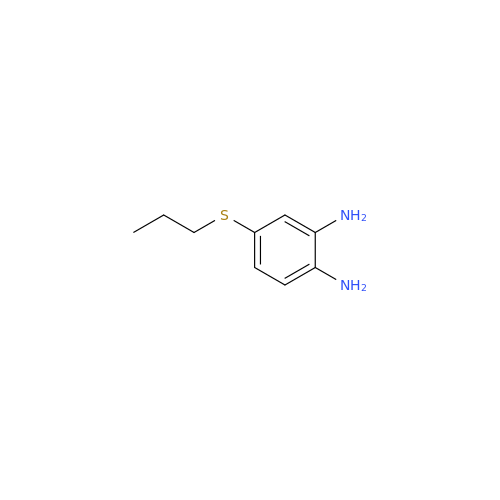
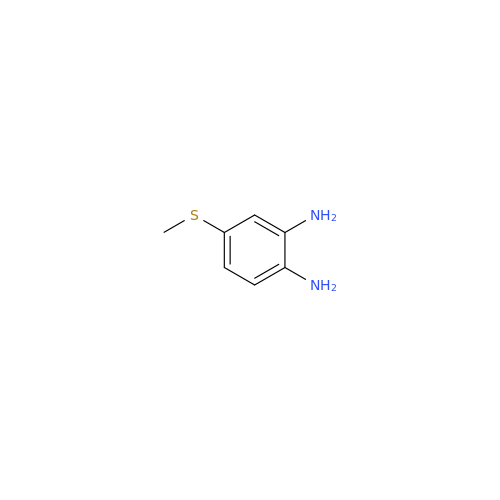
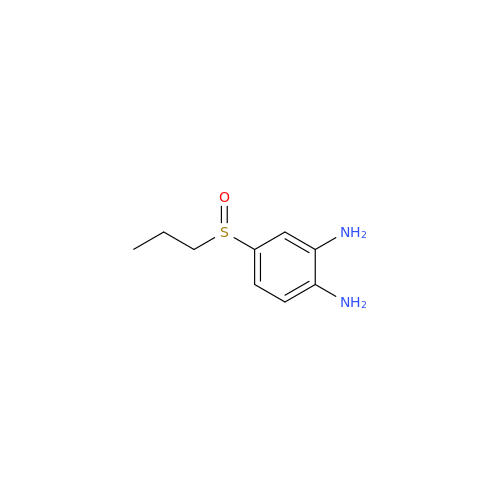
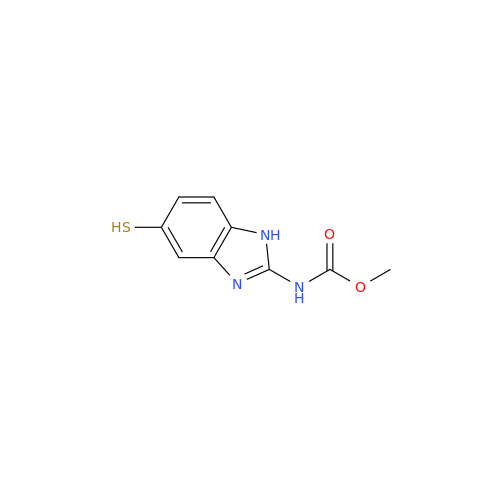
![5-(Propylthio)-1H-benzo[d]imidazole 5-(Propylthio)-1H-benzo[d]imidazole](/uploads/product-details/abd029-5649.png)