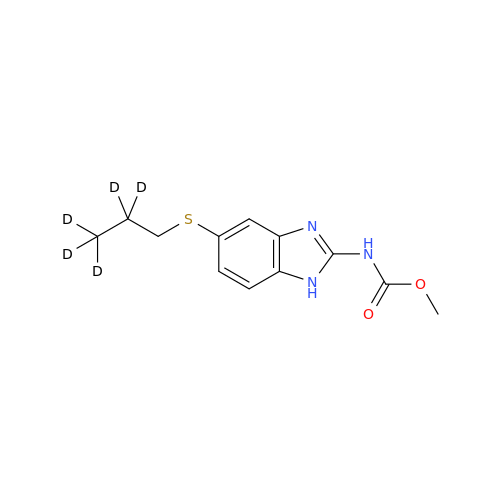
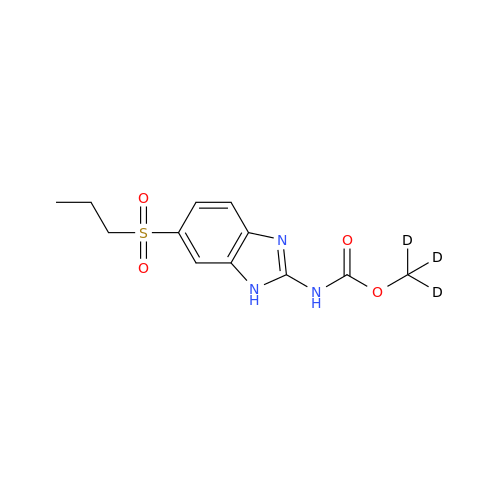
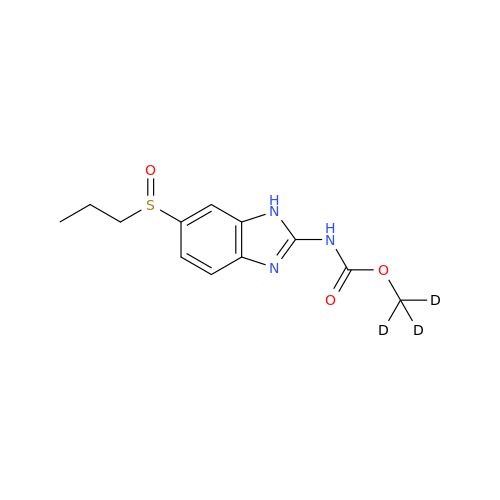
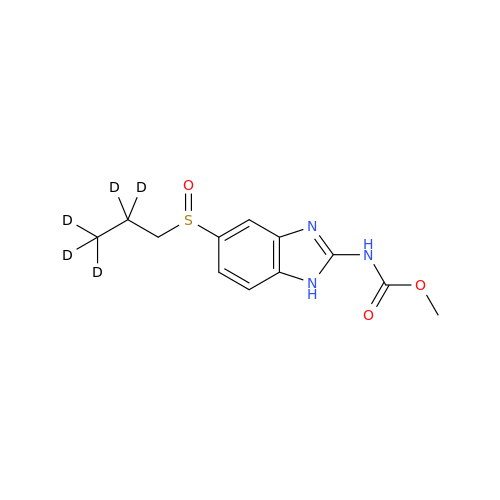
Product Information
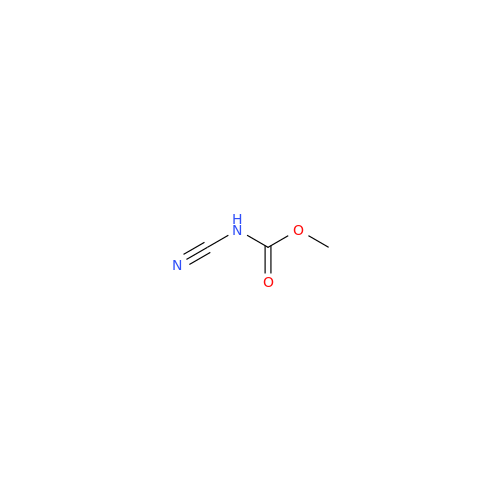
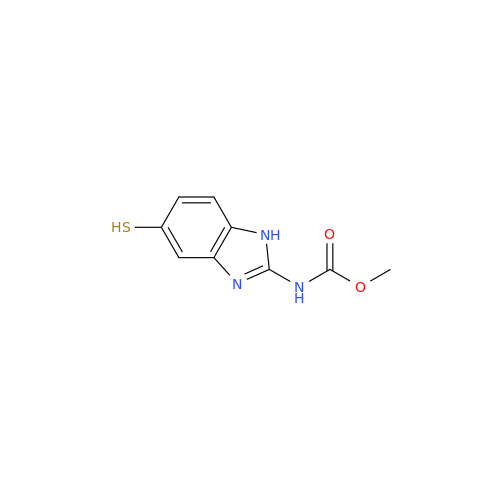
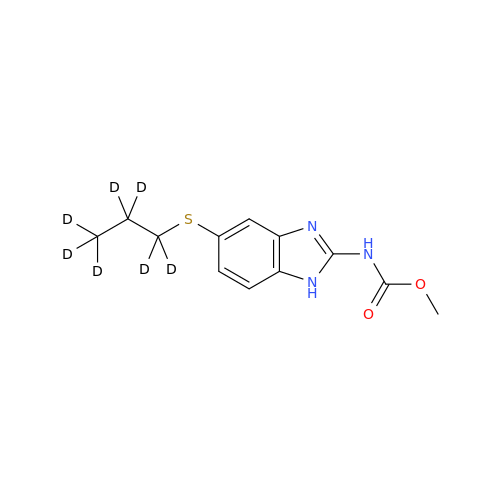
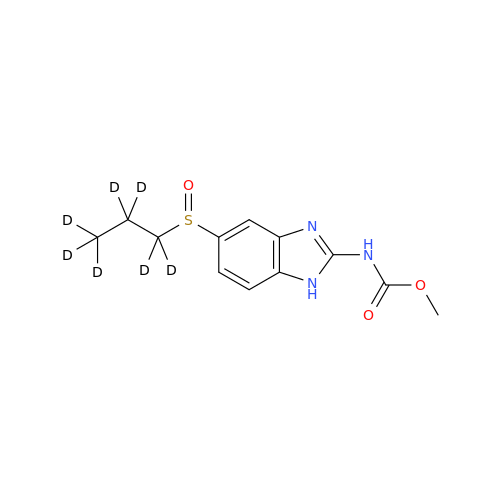
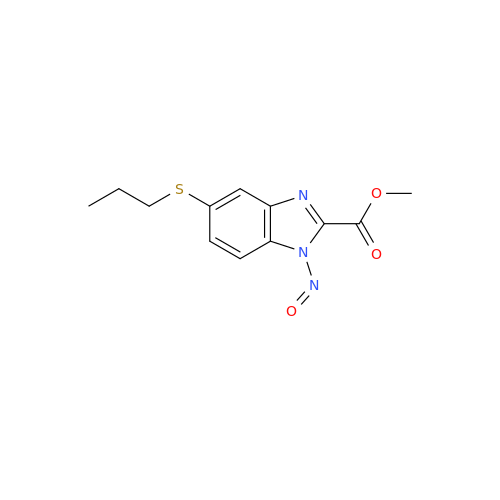
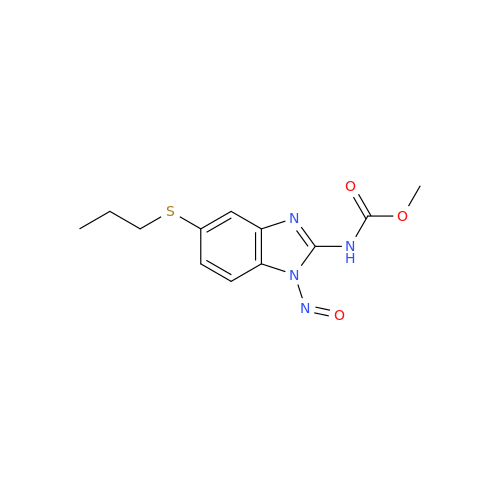
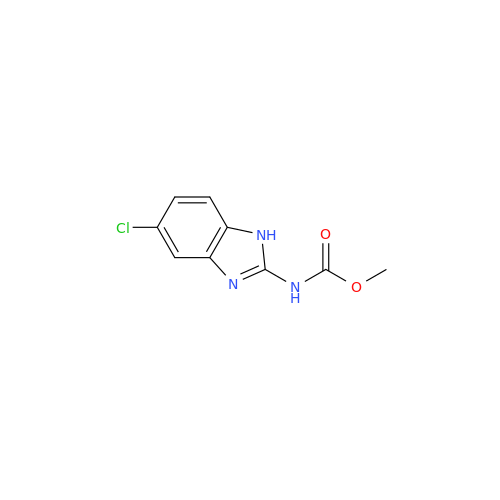
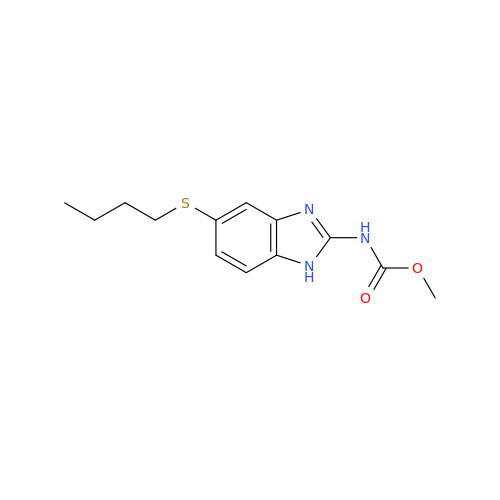
Albendazole EP Impurity G
|
Chemical Name: Albendazole EP Impurity G
Synonym: Methyl (5-chloro-1H-benzo[d]imidazol-2-yl)carbamate; Methyl (5-chloro-1H-benzimidazol-2-yl)carbamate; Febendazole Impurity B (IP); Methyl [5(6)-chlorobenzimidazole-2-yl]carbamate; Fenbendazole Related Compound B (USP)| Enter Batch Number | |||



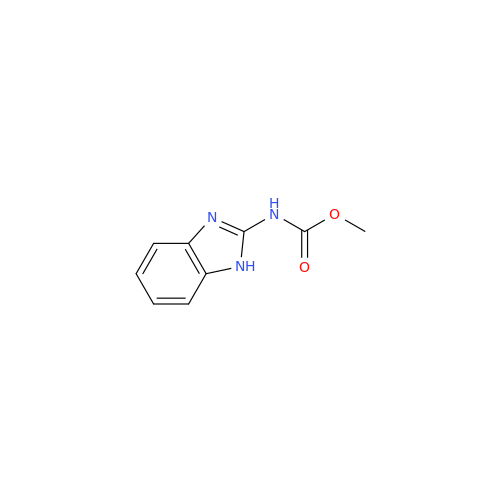
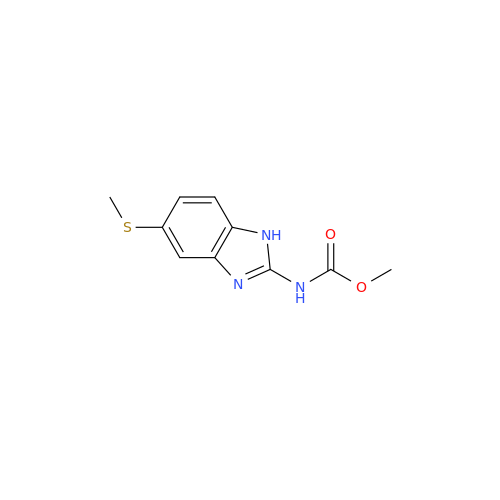
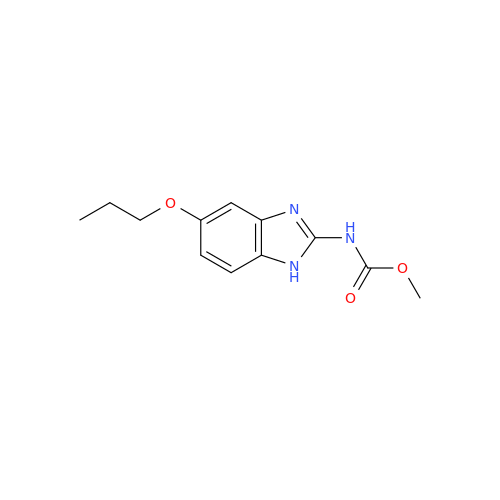
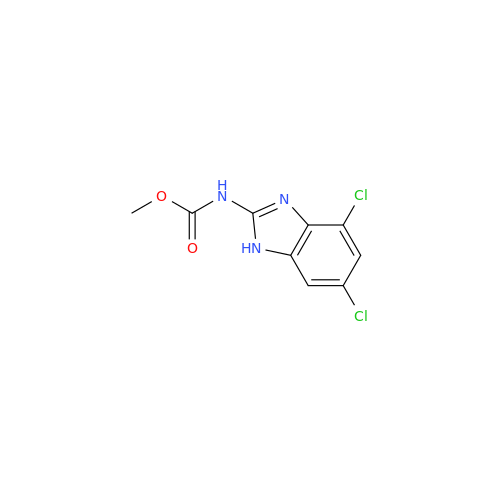
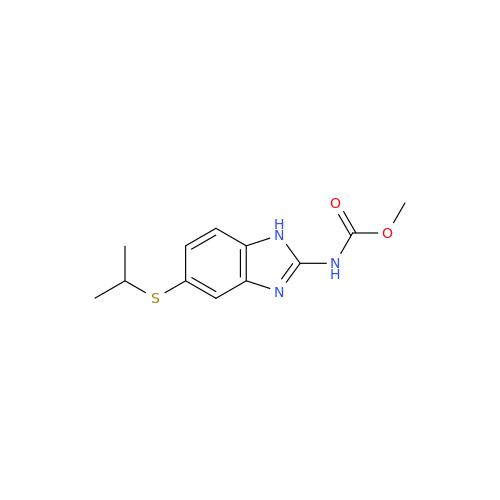
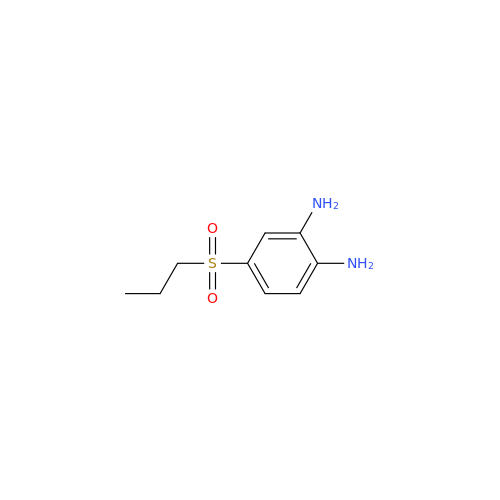
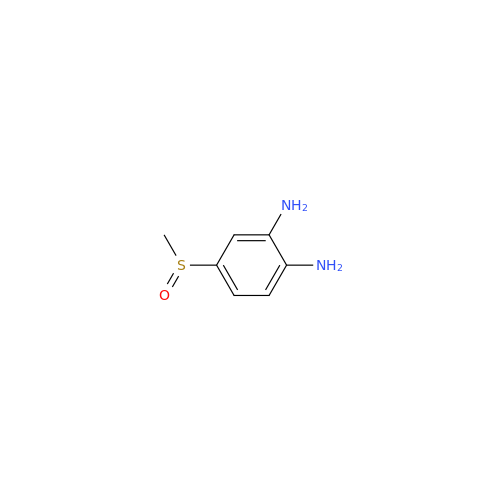
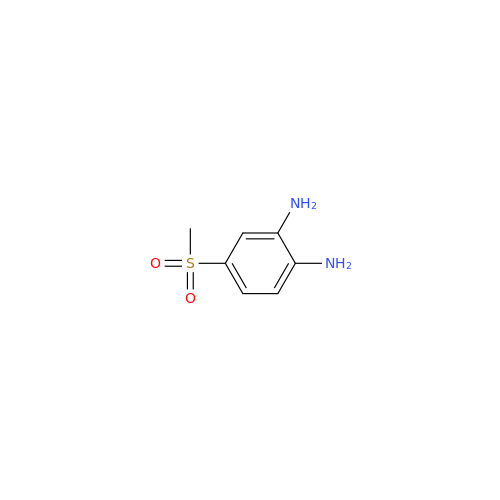
![5-(Propylthio)-1H-benzo[d]imidazole 5-(Propylthio)-1H-benzo[d]imidazole](/uploads/product-details/abd029-5649.png)