Product Information
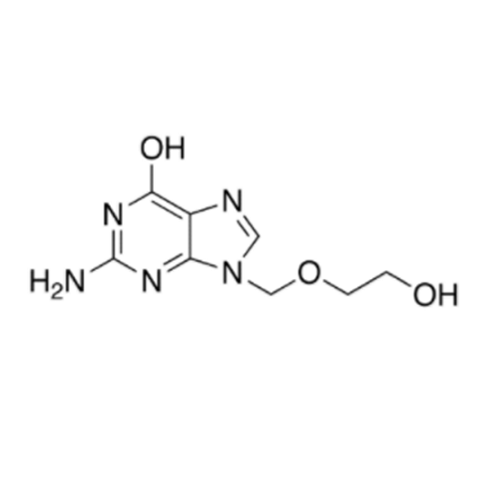
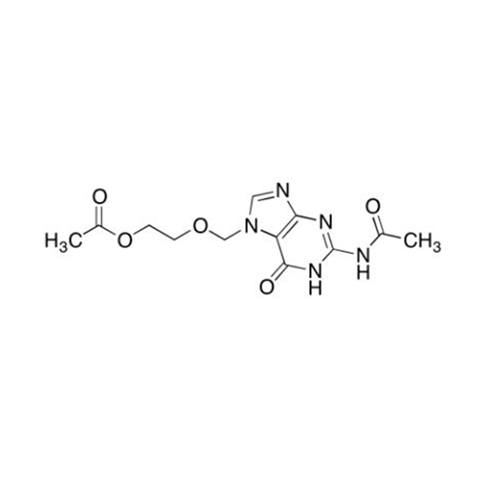
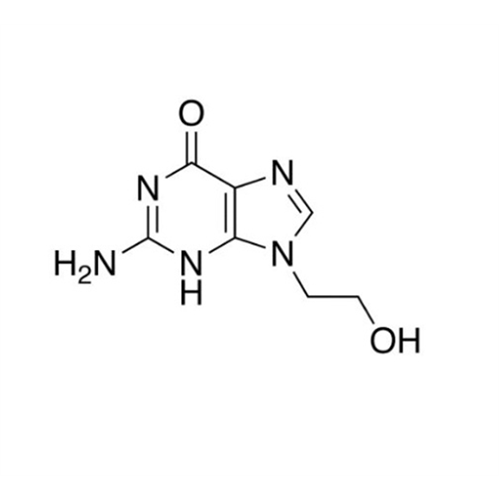
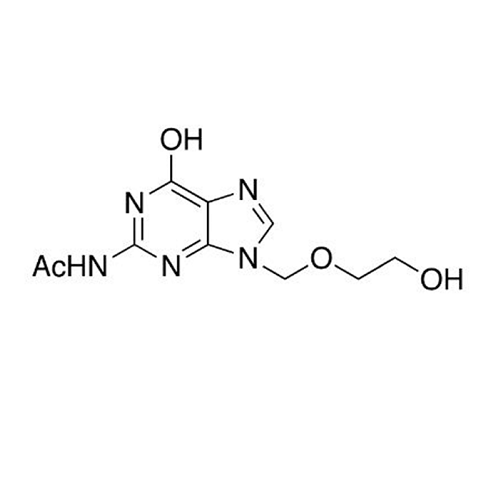
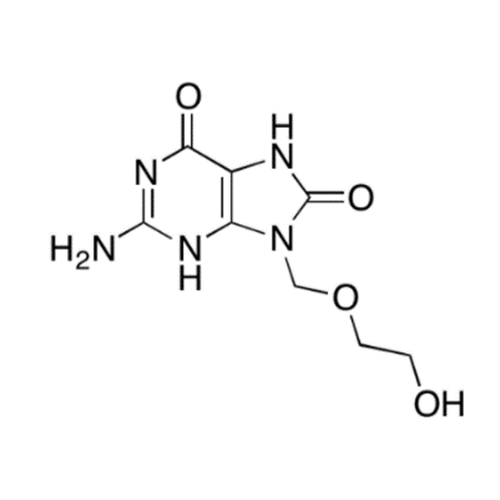
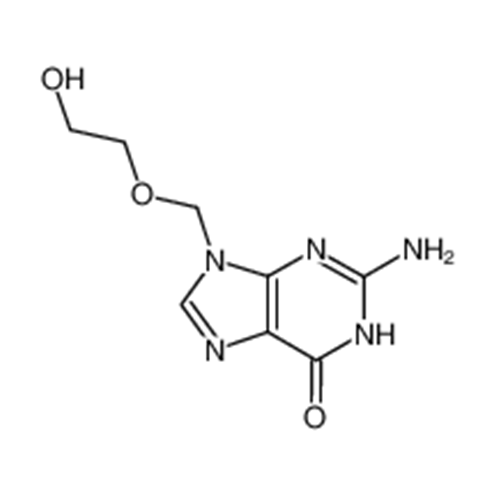
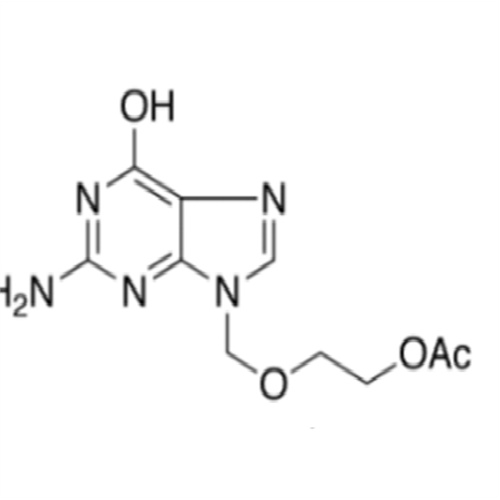
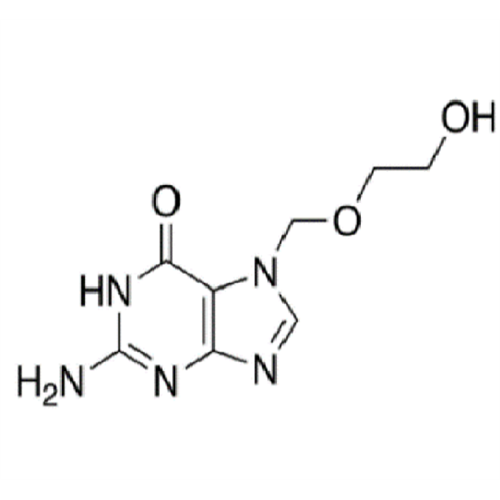
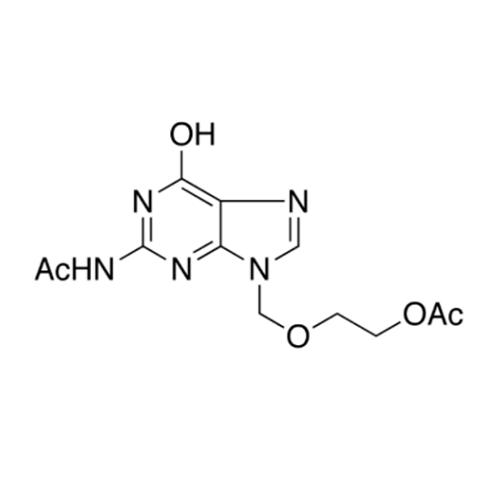
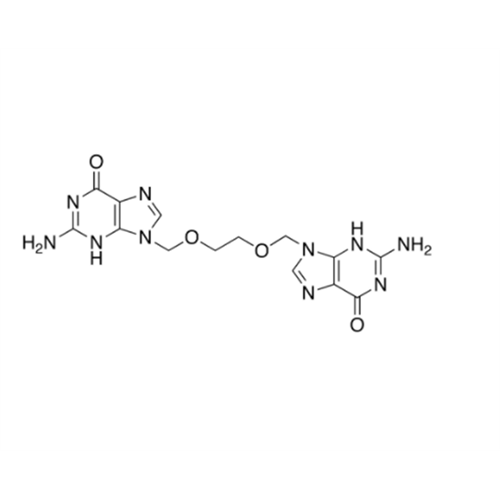
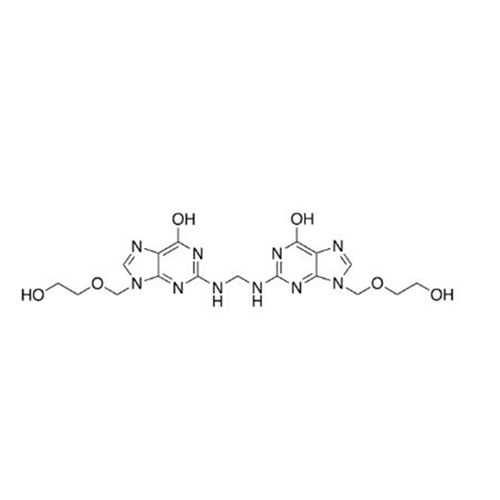
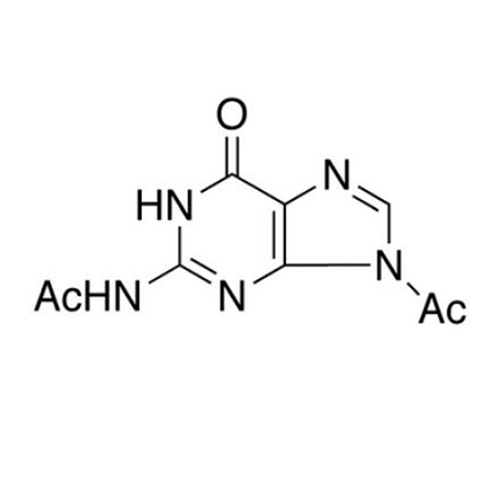
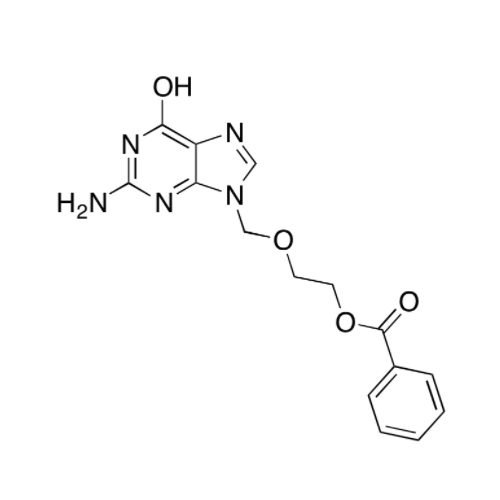
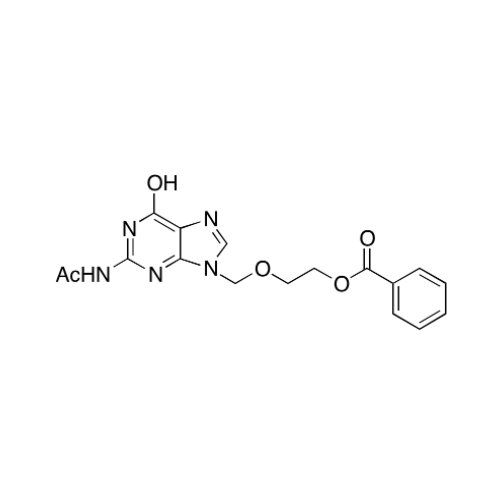
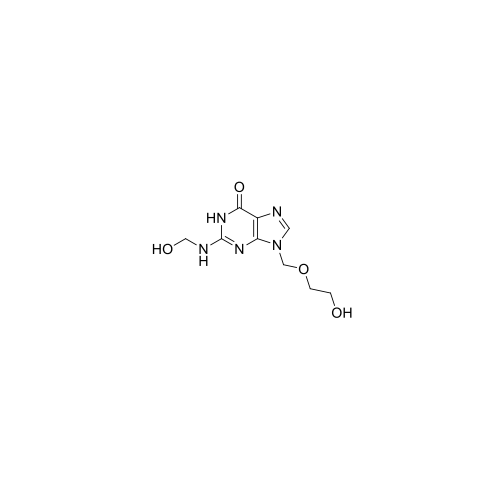
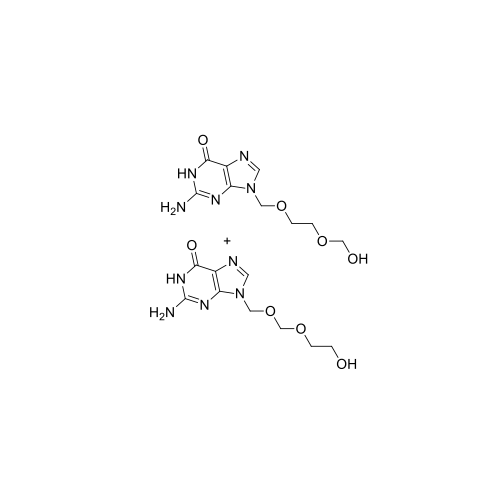
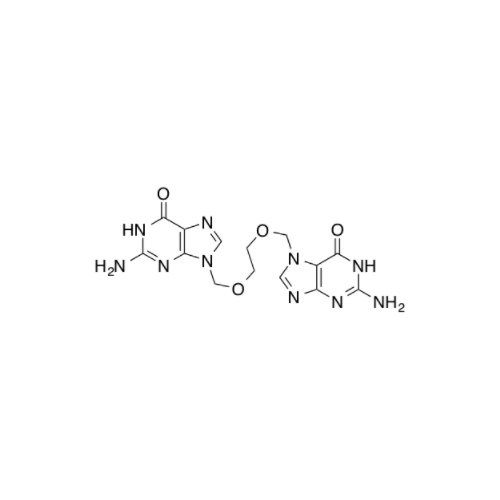
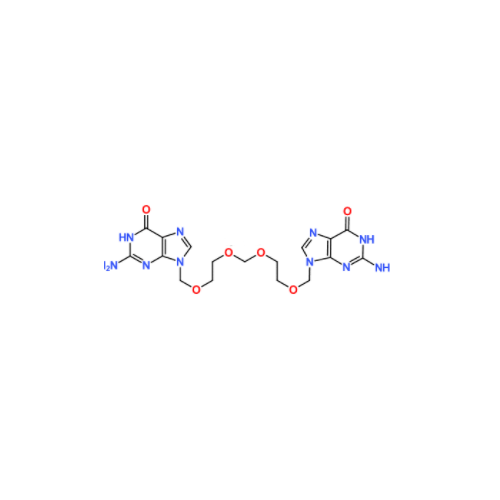
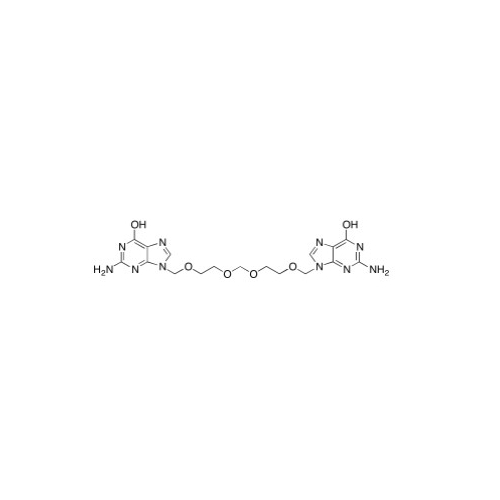
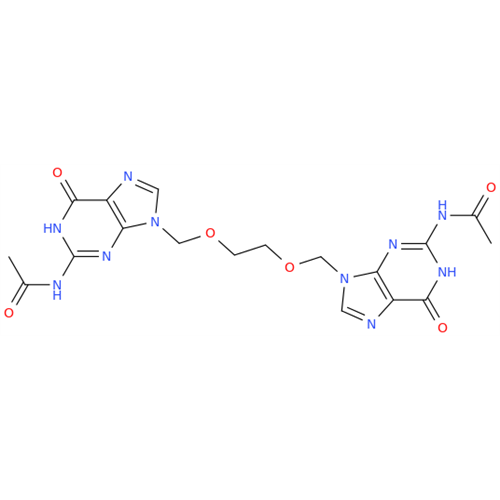
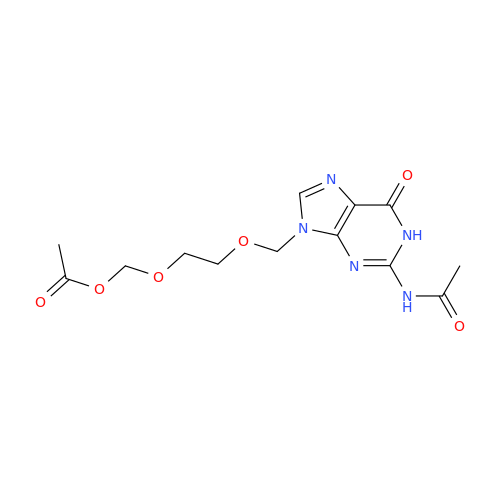
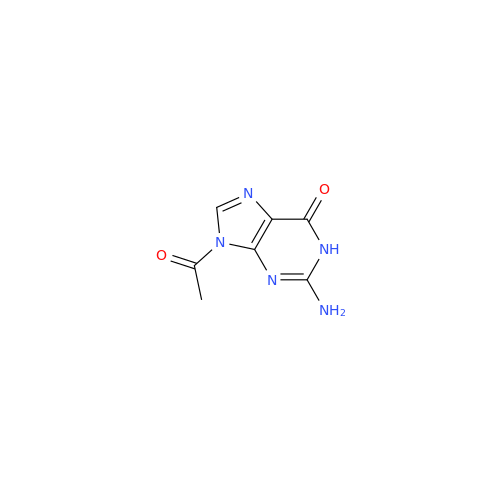
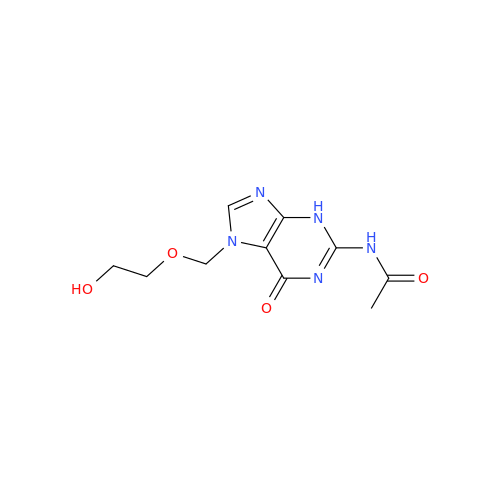
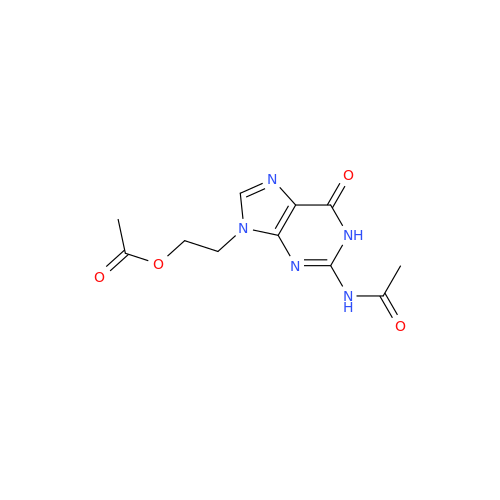
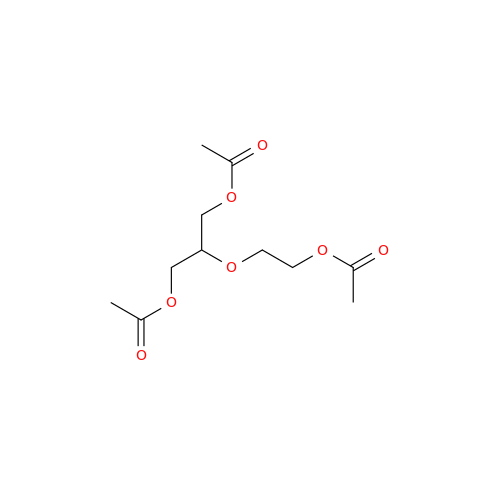
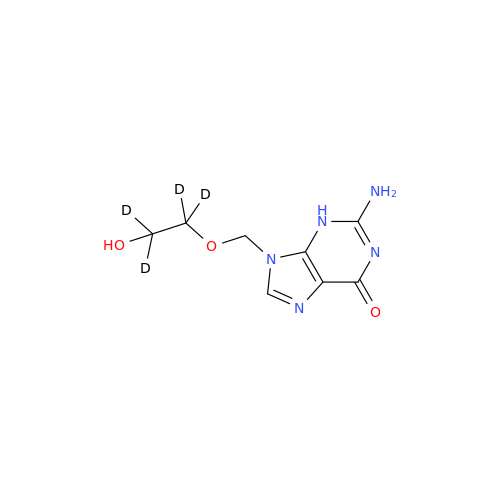
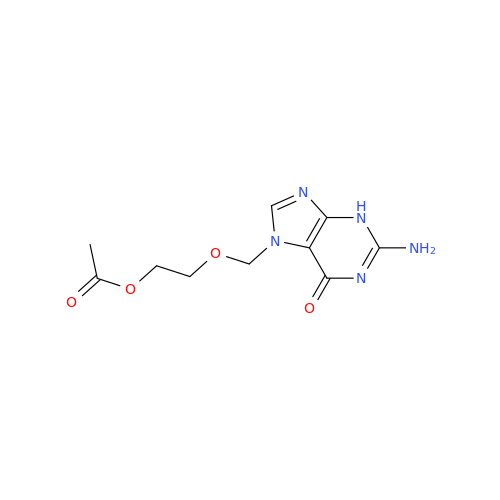
Aciclovir Impurity 14
|
Chemical Name: Aciclovir Impurity 14
Synonym: 6H-Purin-6-one, 7-[[2-(acetyloxy)ethoxy]methyl]-2-amino-1,7-dihydro; 7-[[2-(Acetyloxy)ethoxy]methyl]-2-amino-1,7-dihydro-6H-purin-6-one| Enter Batch Number | |||