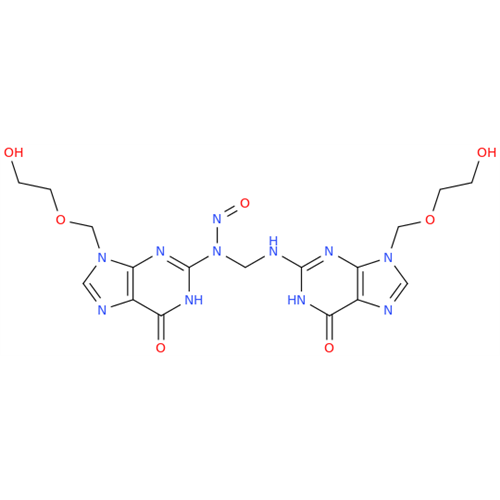
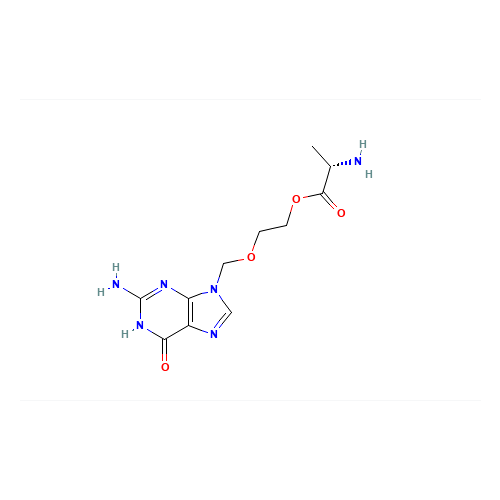
Product Information
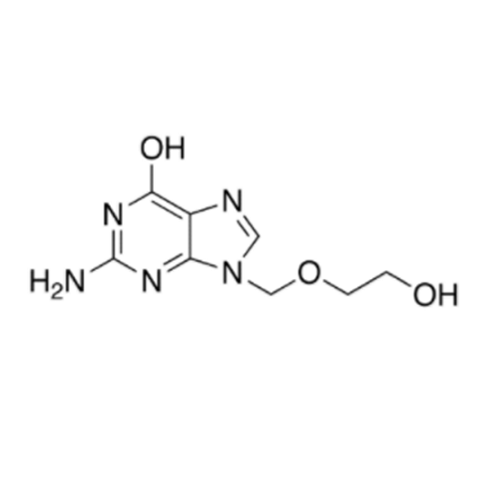
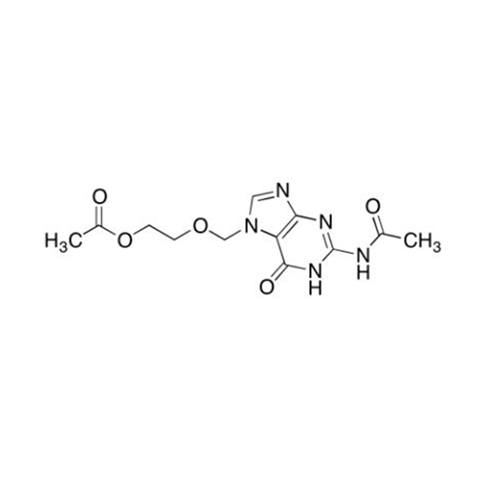
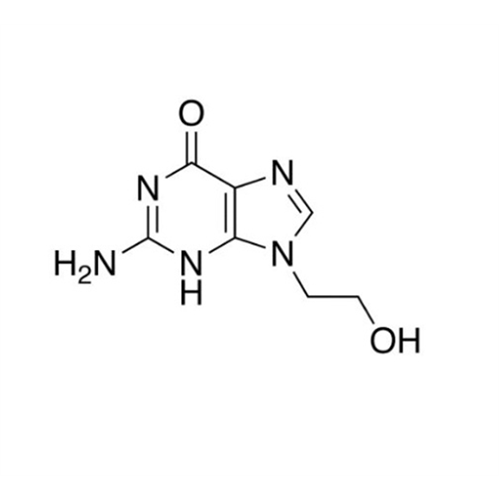
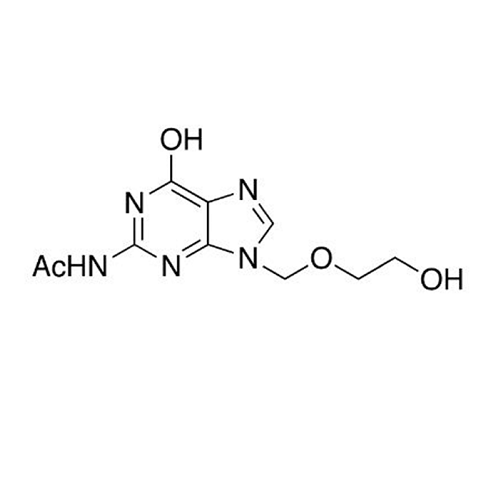
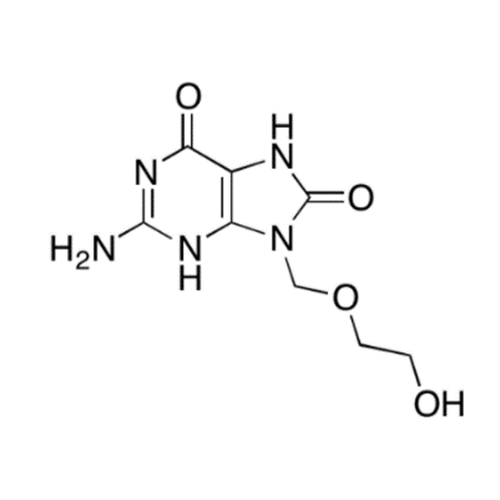
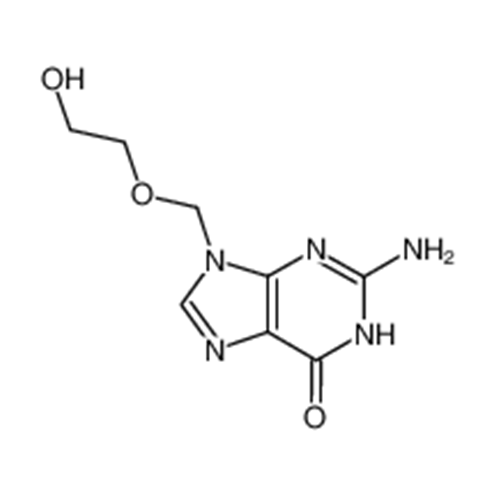
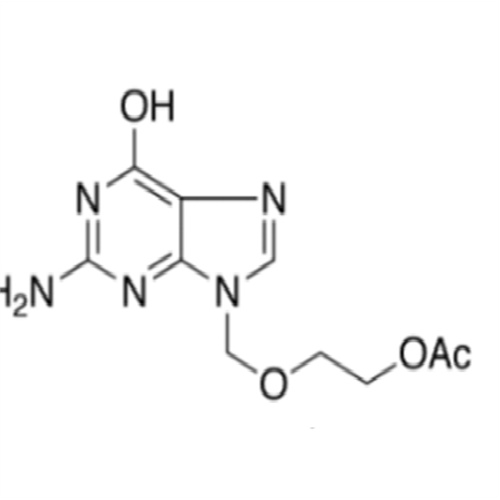
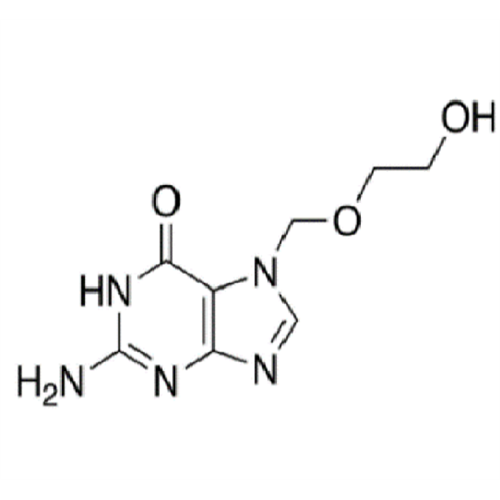
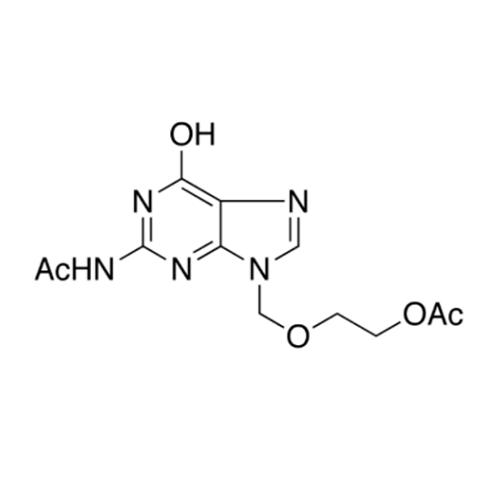
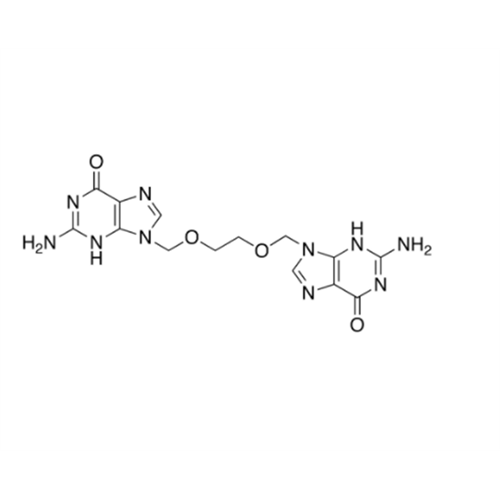
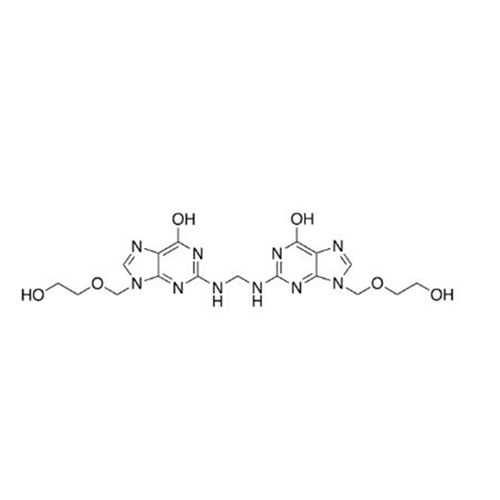
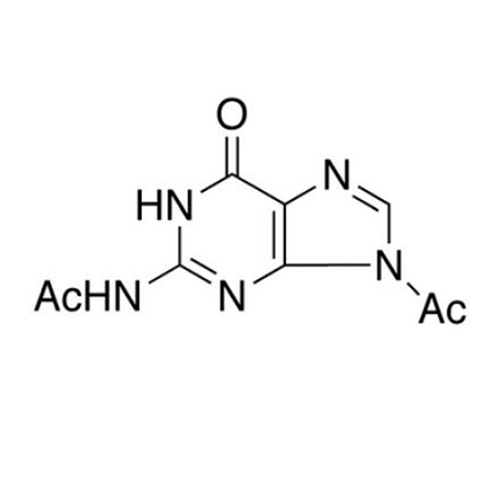
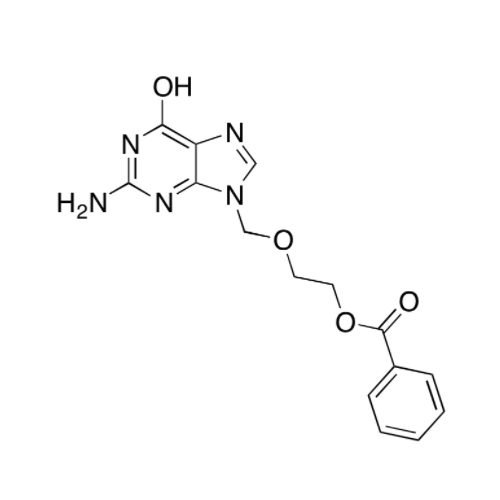
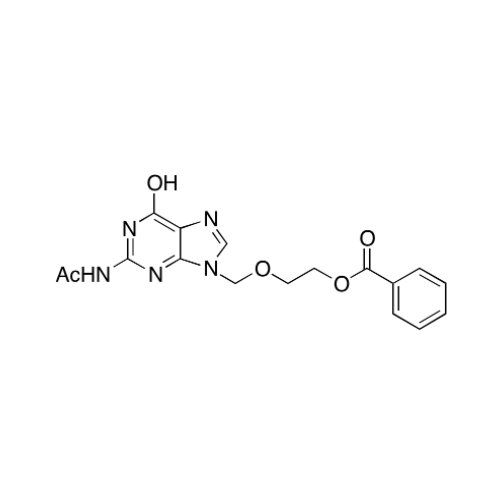
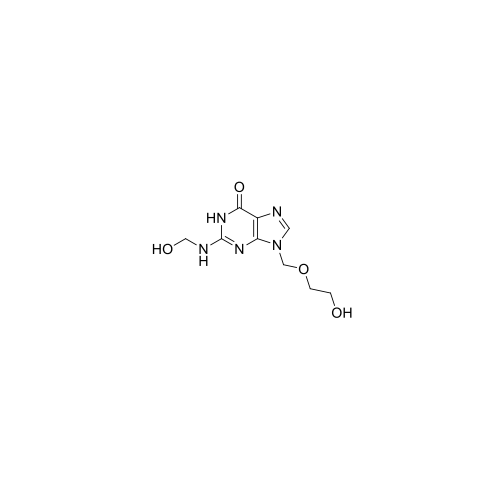
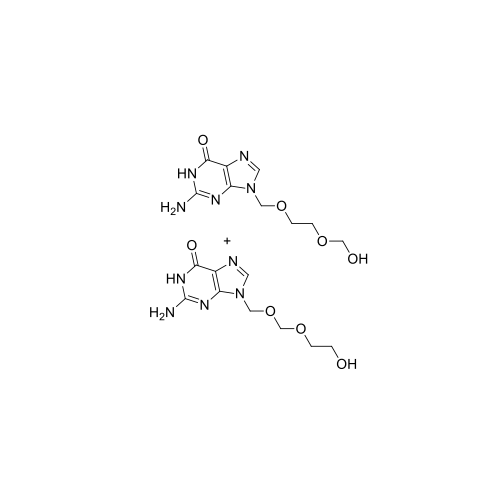
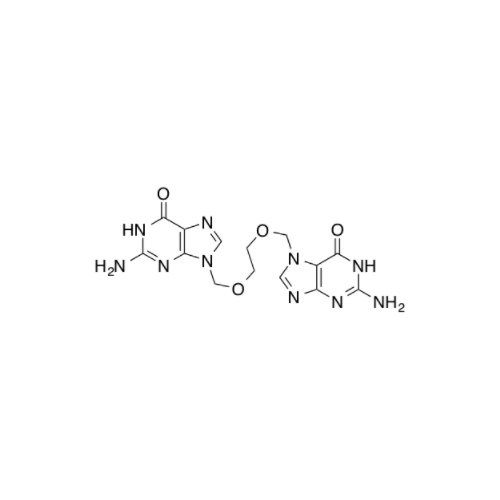
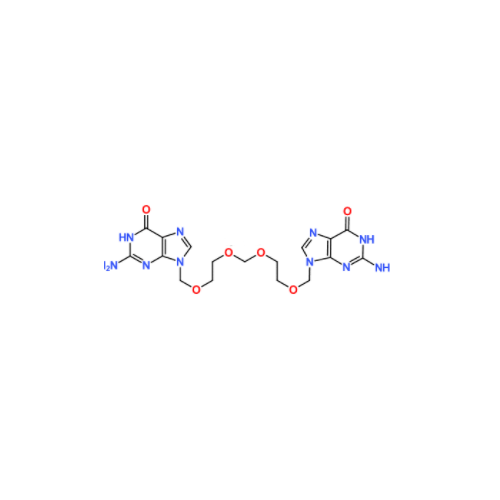
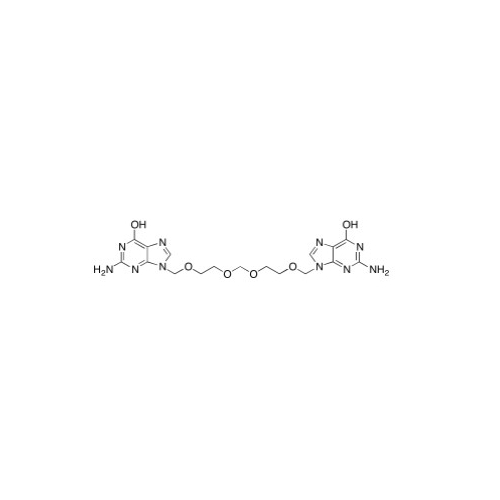
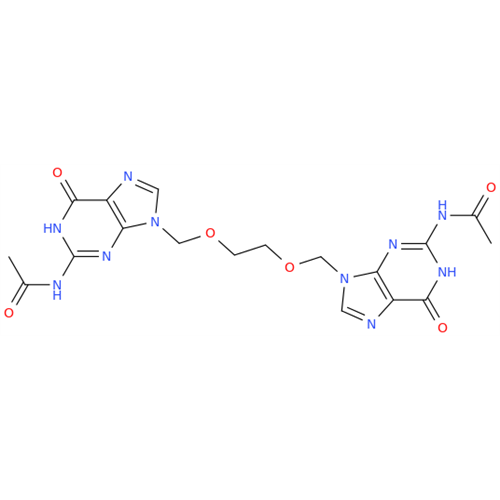
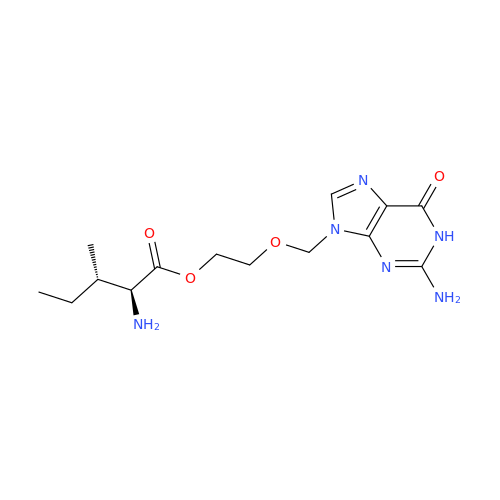
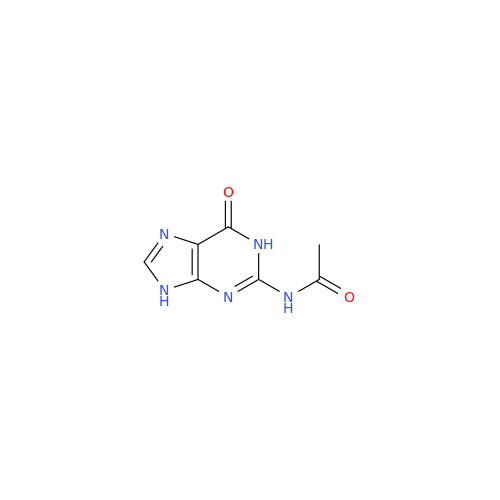
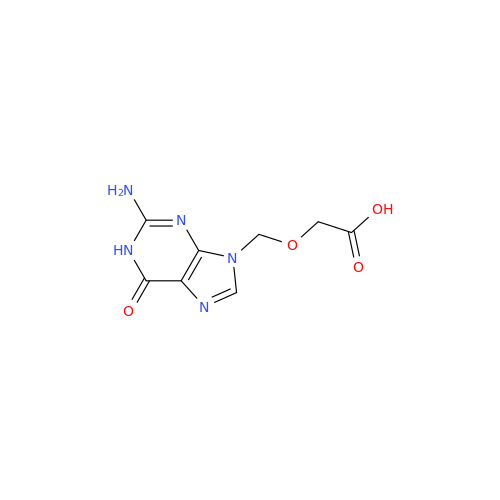
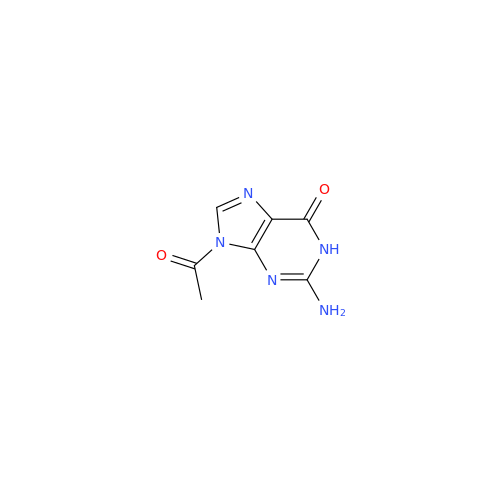
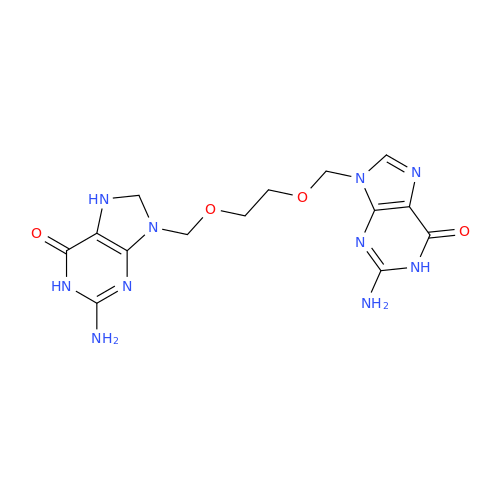
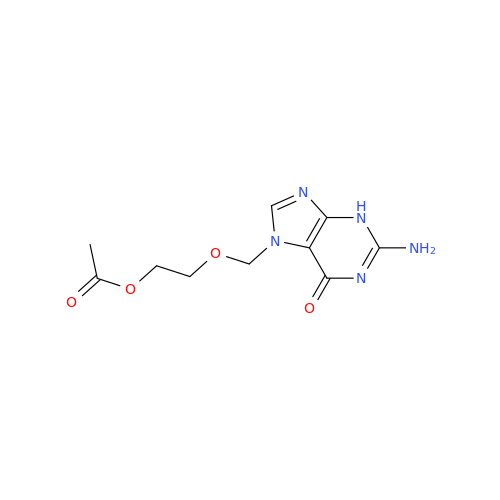
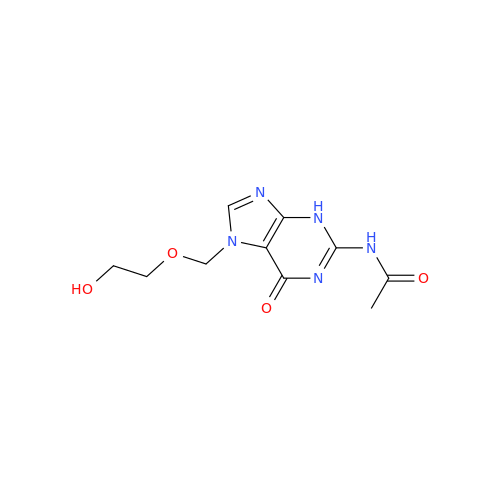
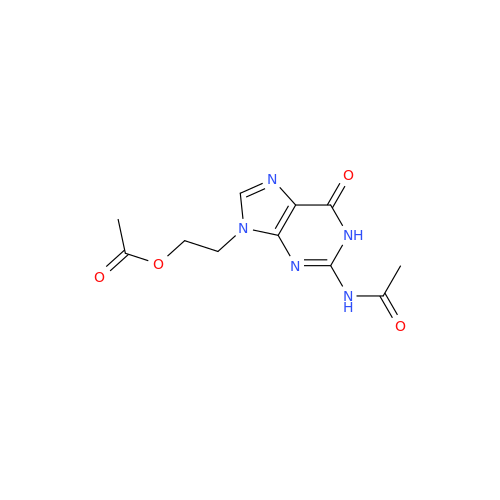
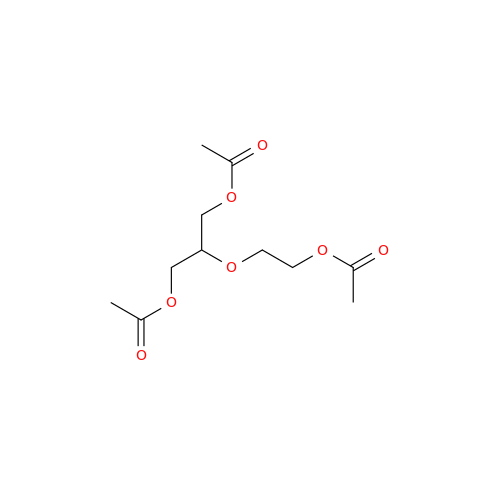
Aciclovir Impurity N
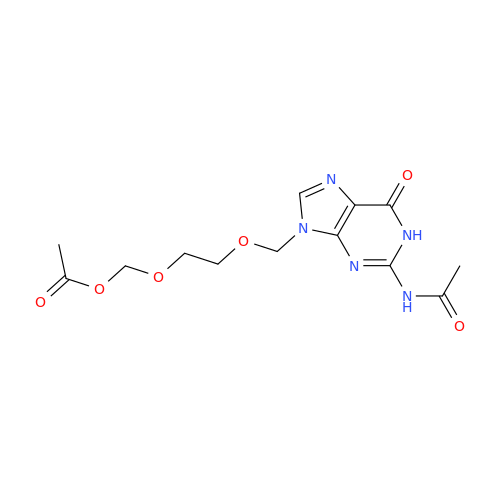
|
Chemical Name: Aciclovir Impurity N
Synonym: (2-((2-acetamido-6-oxo-1,6-dihydro-9H-purin-9-yl)methoxy)ethoxy)methyl acetate; Acyclovir - In House Impurity| Enter Batch Number | |||