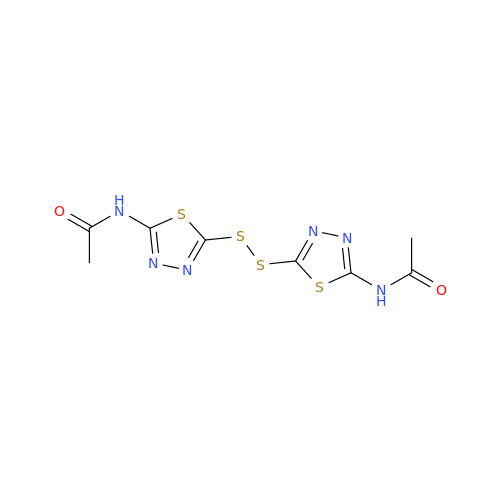
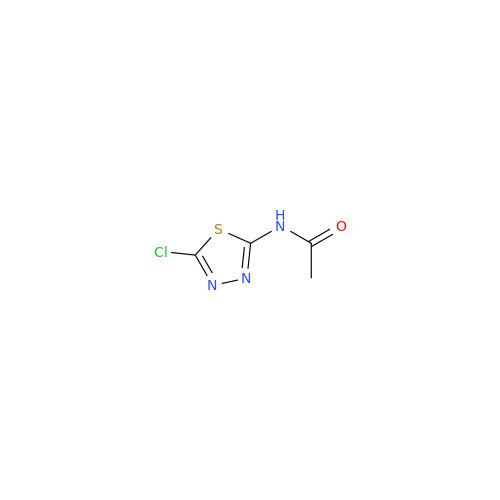
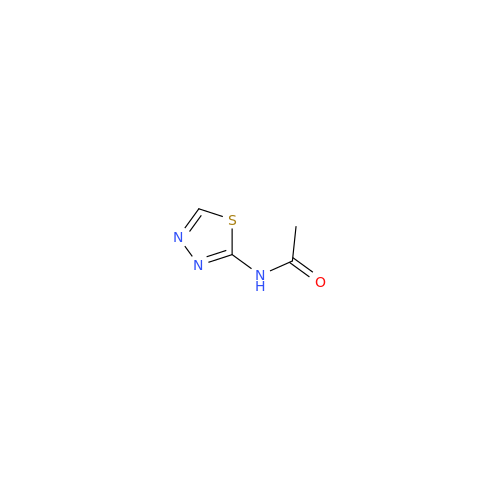
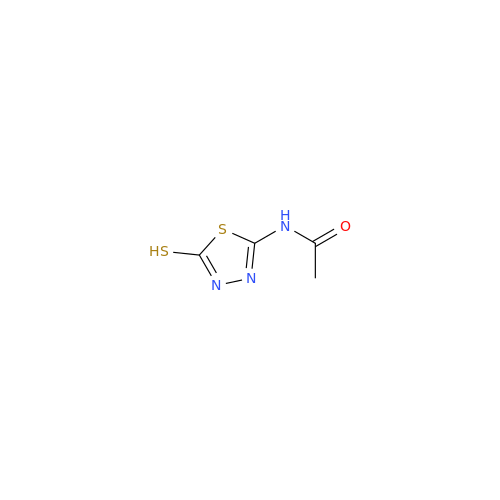
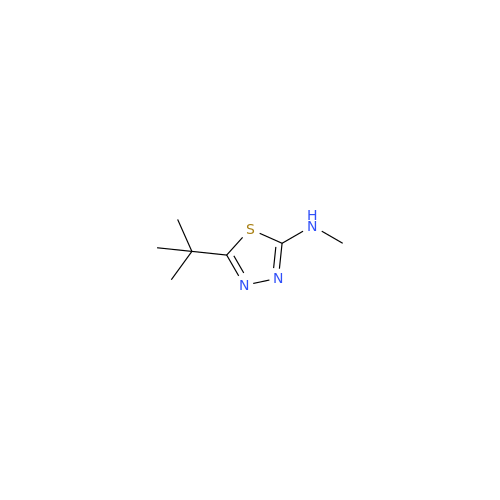
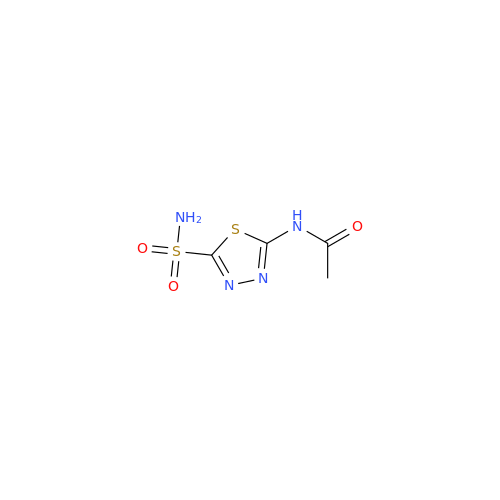
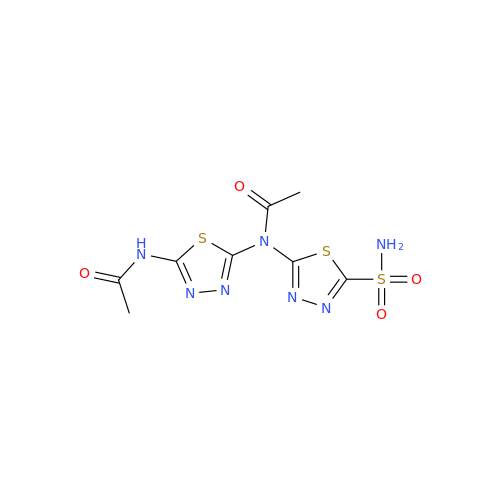
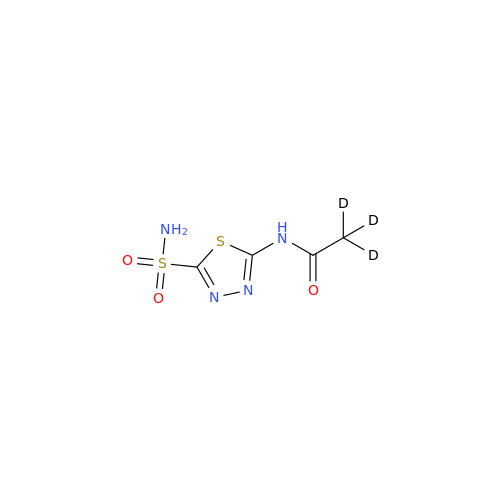
Product Information
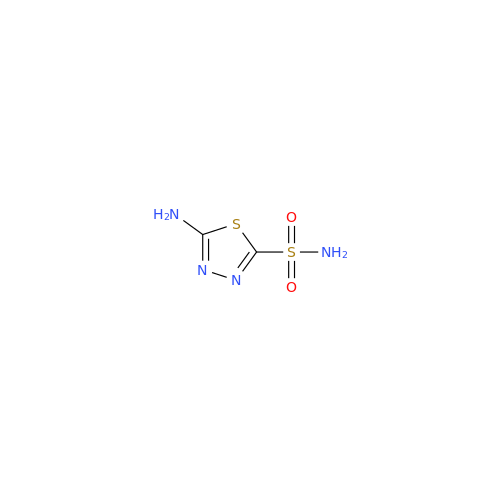
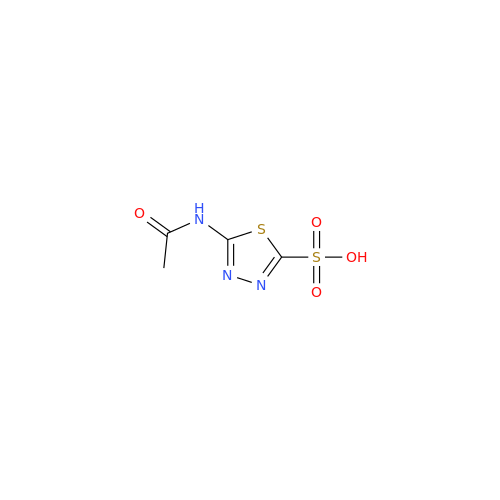
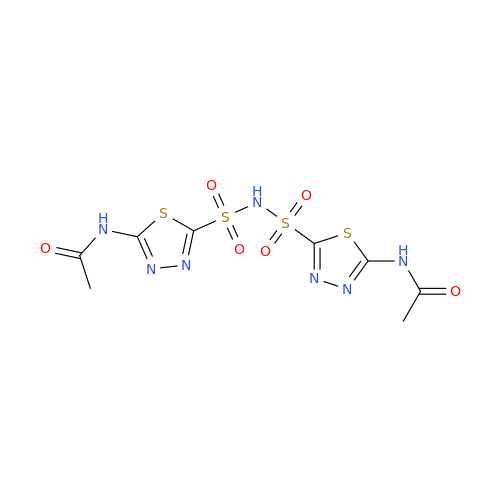
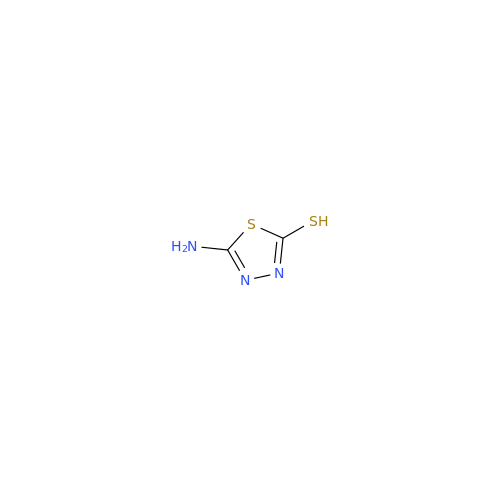
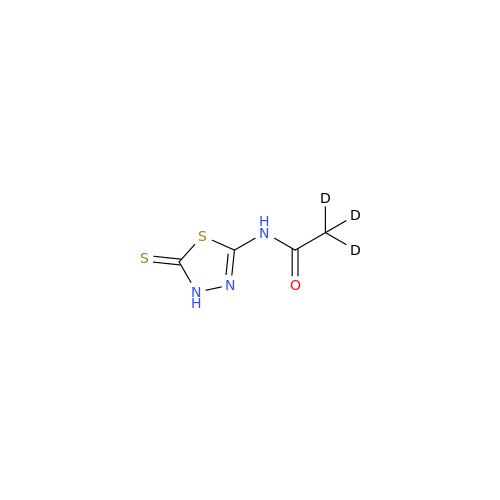
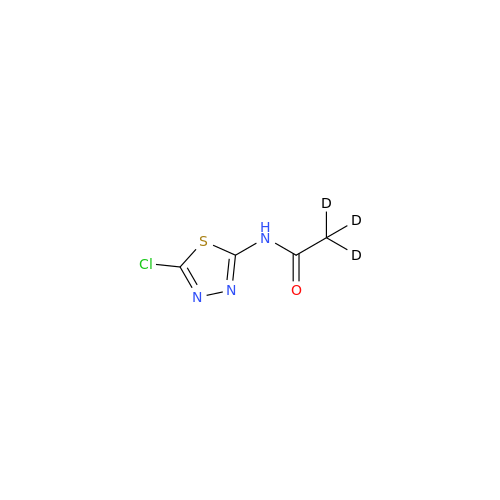
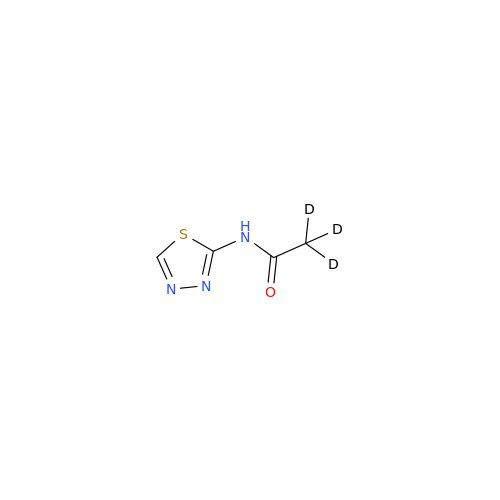
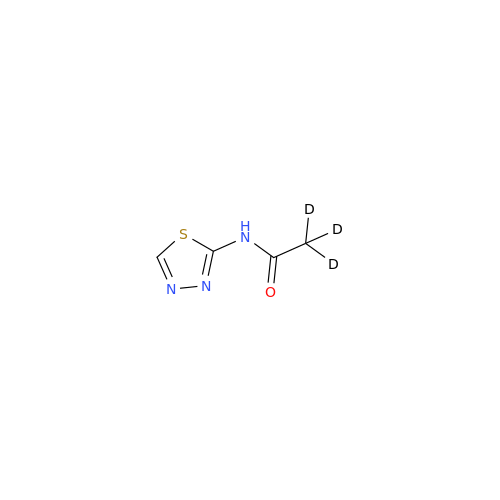
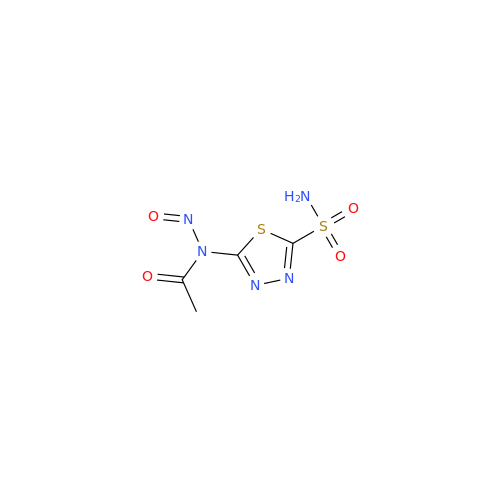
Acetazolamide N-Nitroso Impurity
|
Chemical Name: Acetazolamide N-Nitroso Impurity
Synonym: Acetazolamide N-Nitroso Impurity| Enter Batch Number | |||