Product Information
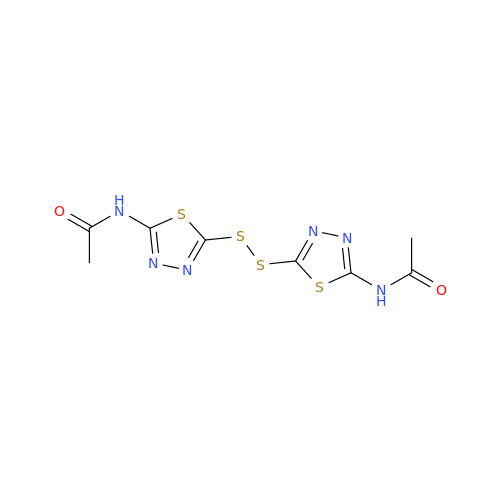
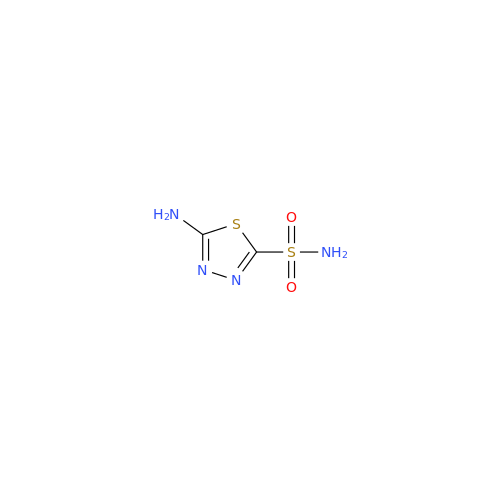
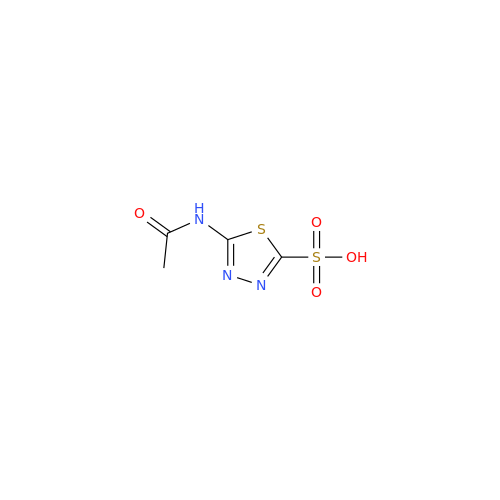
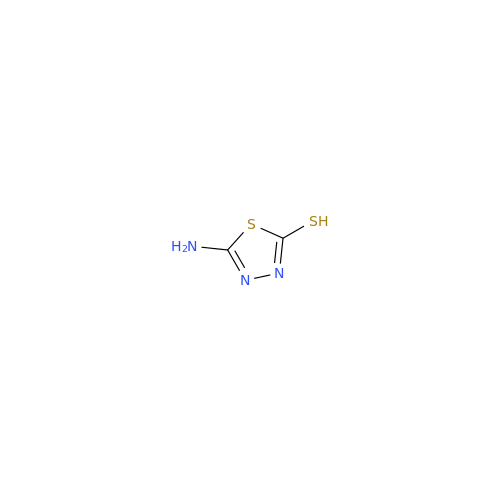
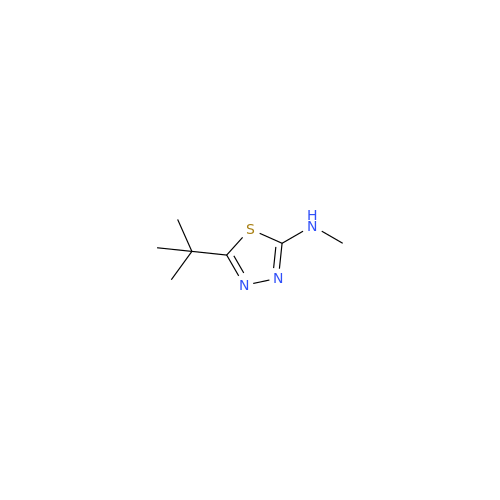
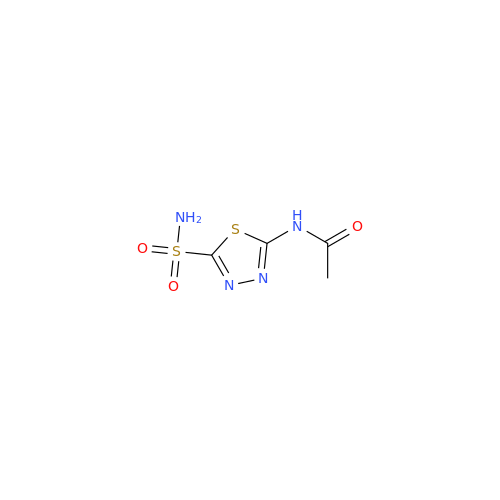
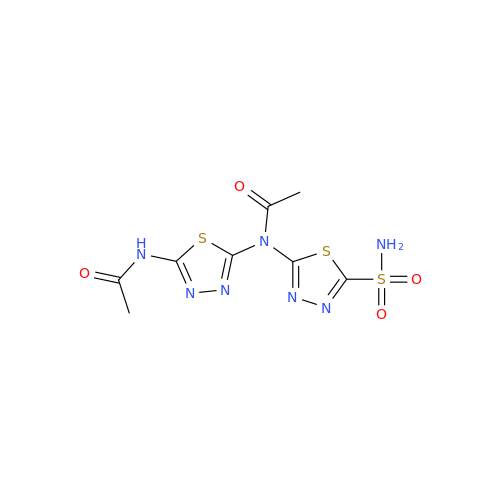
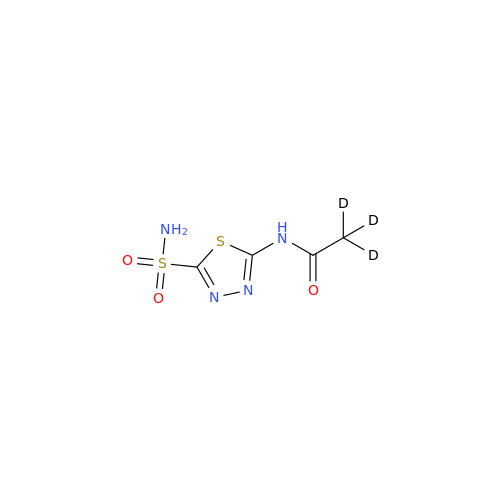
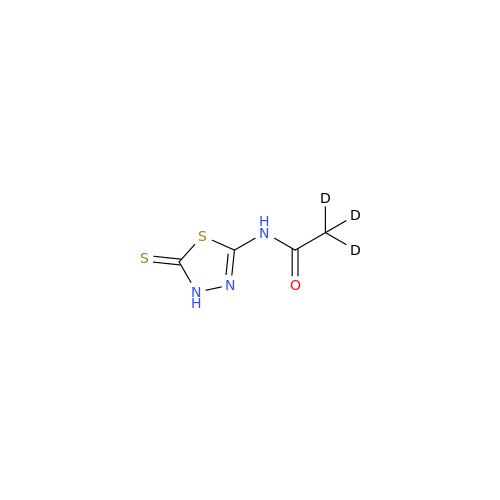
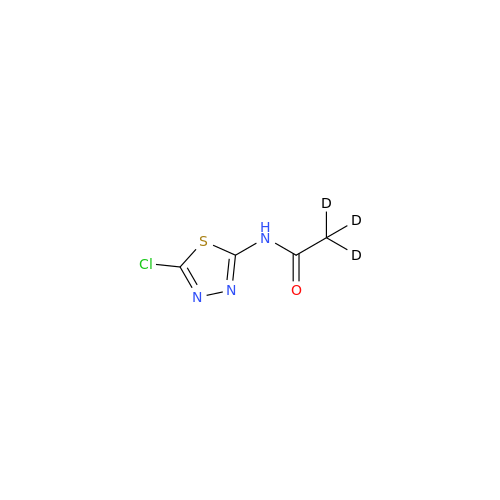
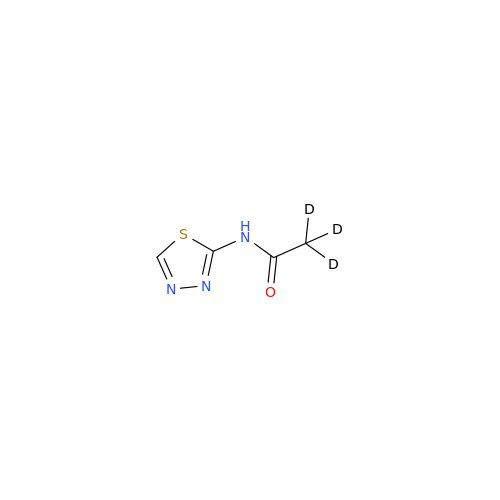
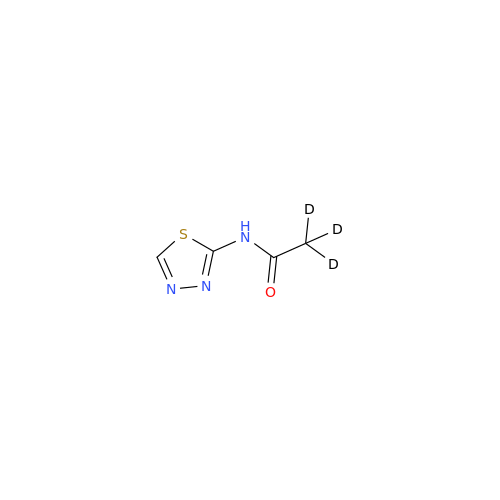
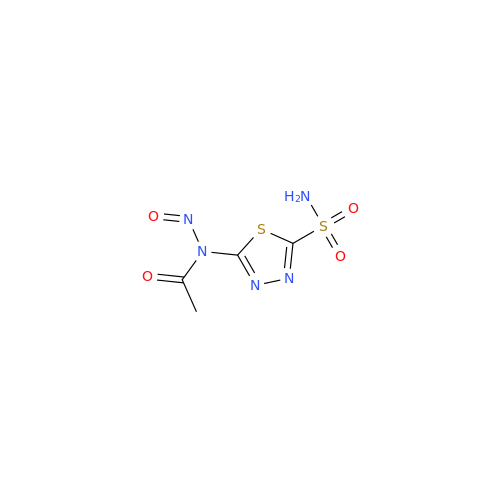
Acetazolamide EP Impurity F
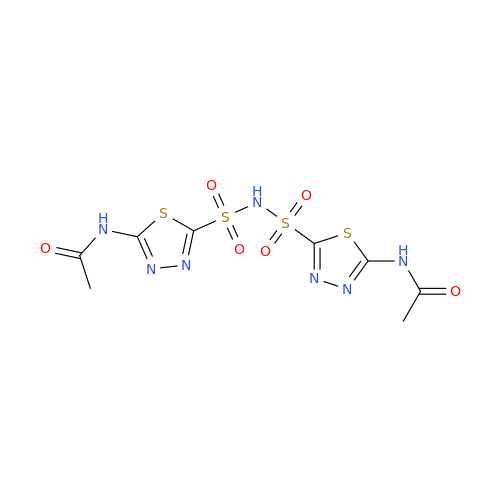
|
Chemical Name: Acetazolamide EP Impurity F
Synonym: Bis[5-(acetylamino)-1,3,4-thiadiazole-2-sulfonyl]amine; N-(5-(N-((5-acetamido-1,3,4-thiadiazol-2-yl)thio)sulfamoyl)-1,3,4-thiadiazol-2-yl)acetamide (Impurity - E As per EP); N,N'-[Iminobis(sulfonyl-1,3,4-thiadiazole-5,2-diyl)]bisacetamide; Acetazolamide Dimer; N,N′-{5,5′-[(Hydrosulfonylamino)sulfonyl]bis(1,3,4-thiadiazole-5,2-diyl)}diacetamide (USP)| Enter Batch Number | |||