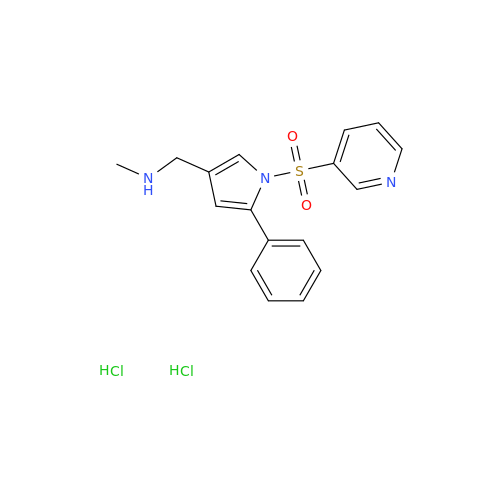
Product Information
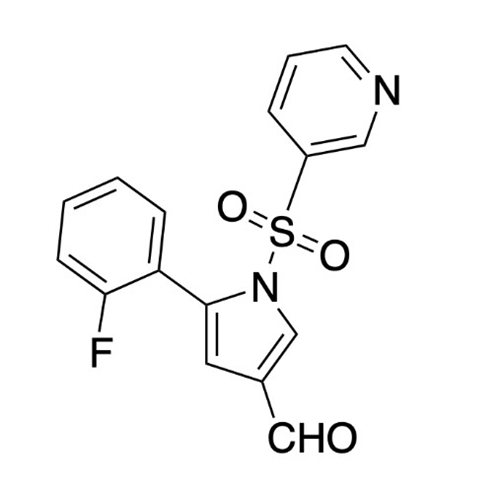
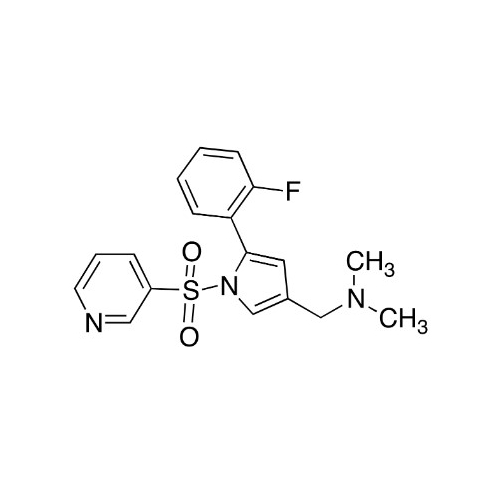
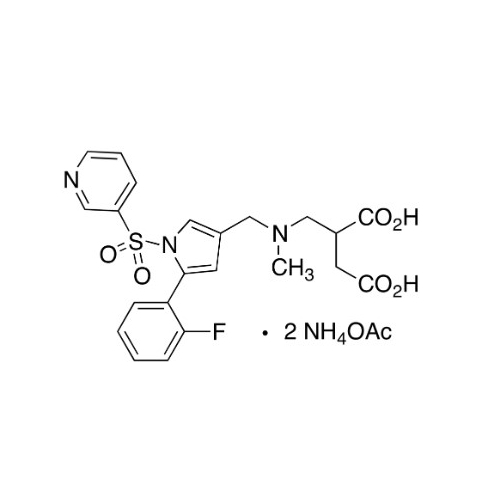
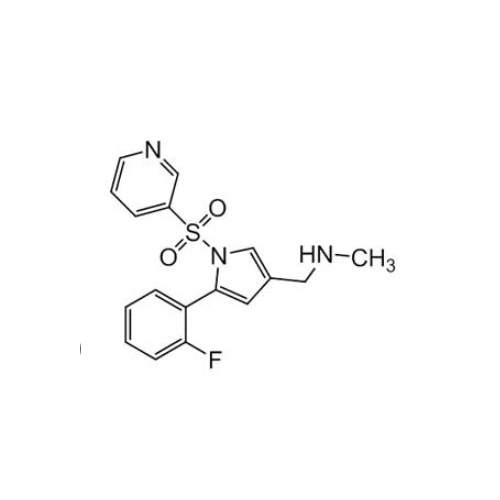
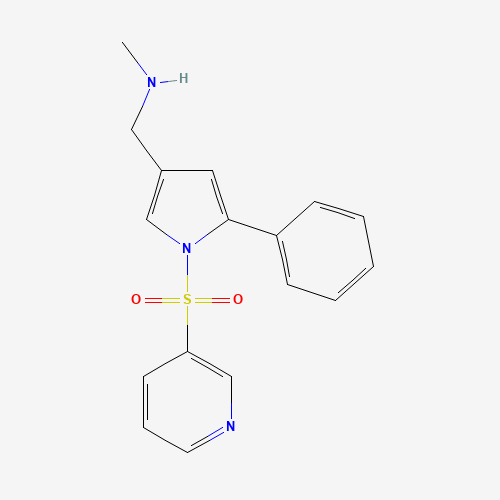
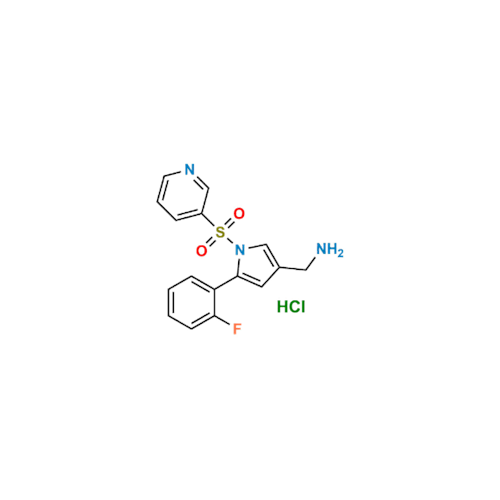
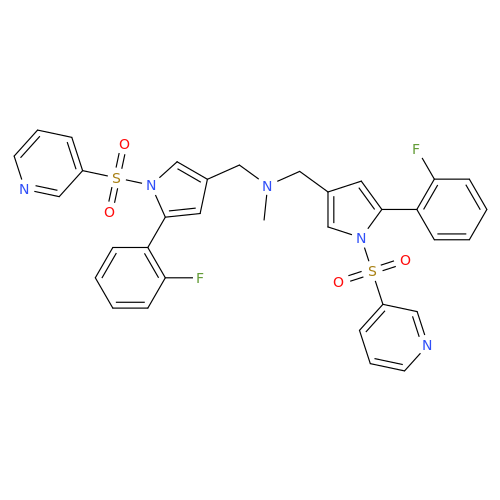
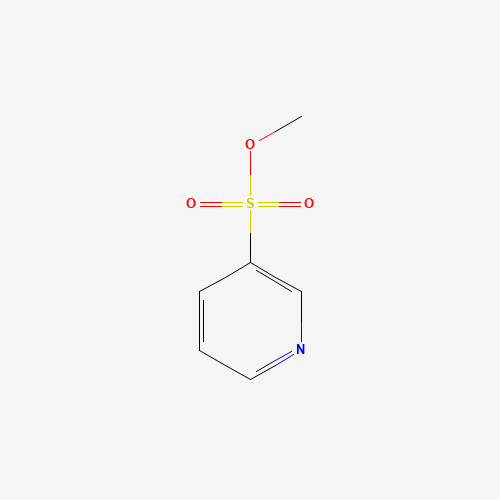
Vonoprazan Impurity 19
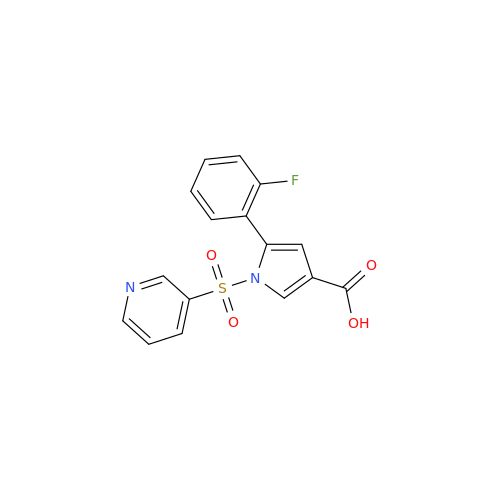
|
Chemical Name: Vonoprazan Impurity 19
Synonym: H-Pyrrole-3-carboxylic acid, 5-(2-fluorophenyl)-1-(3-pyridinylsulfonyl); Vonoprazan M-l metabolite| Enter Batch Number | |||