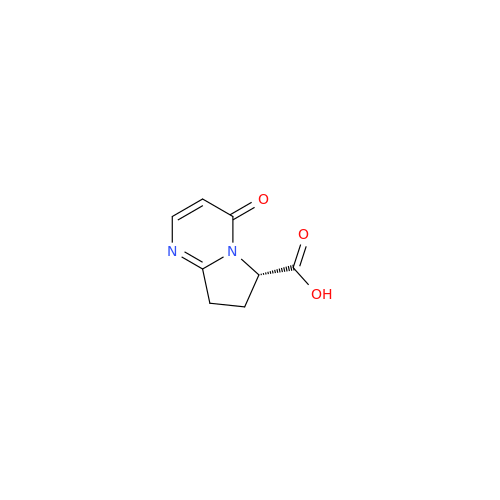
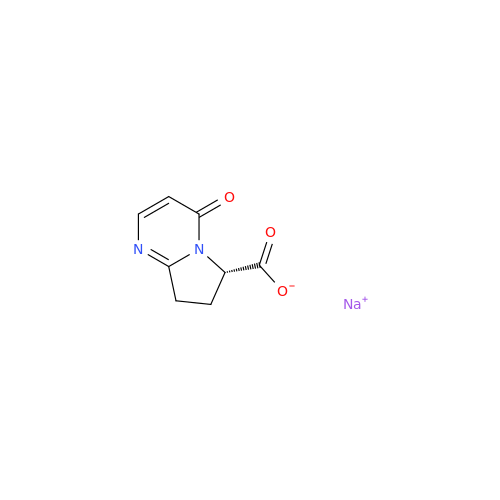
Product Information
Vibegron Impurity 9

|
Chemical Name: Vibegron Impurity 9
Synonym: Benzenemethanol,alpha-[(1R)-1-amino-5-(4-nitrophenyl)-4-pentyn-1-yl]-,hydrochloride| Enter Batch Number | |||