Product Information
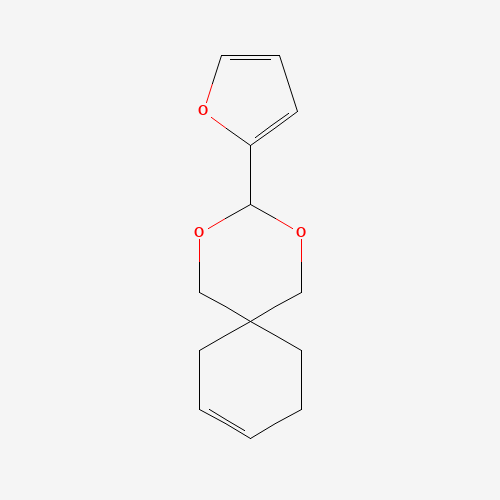
Ulinastatin
|
Chemical Name: Ulinastatin
Synonym: Acid-stable protease inhibitor; Uristatin; Urinary trypsin inhibitor (68)| Enter Batch Number | |||


