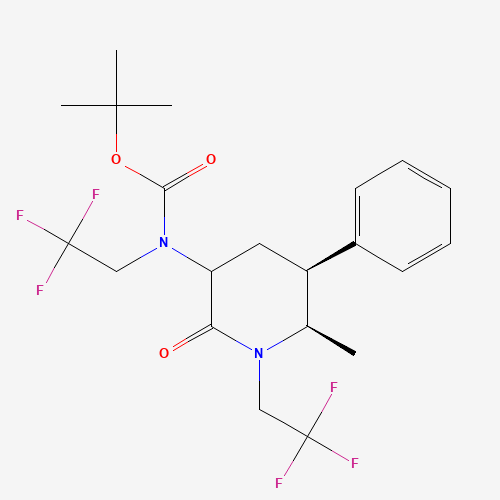
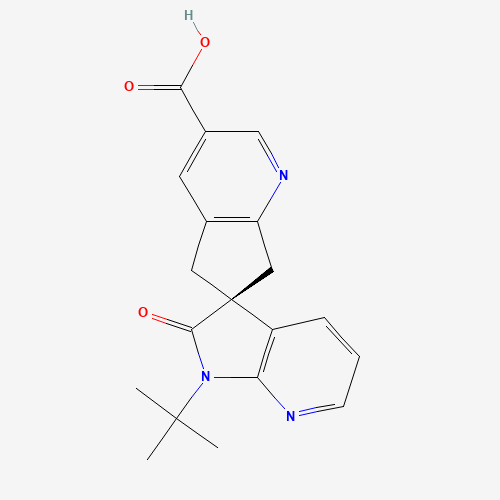
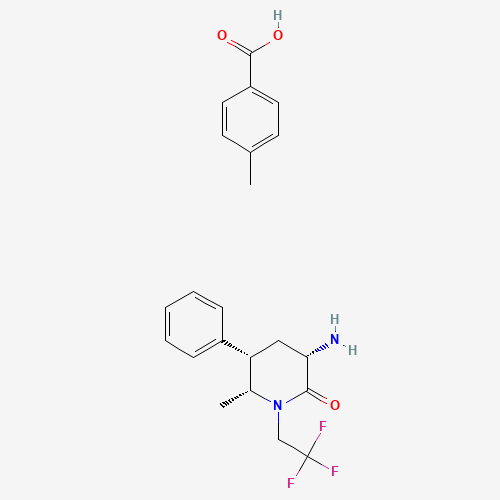
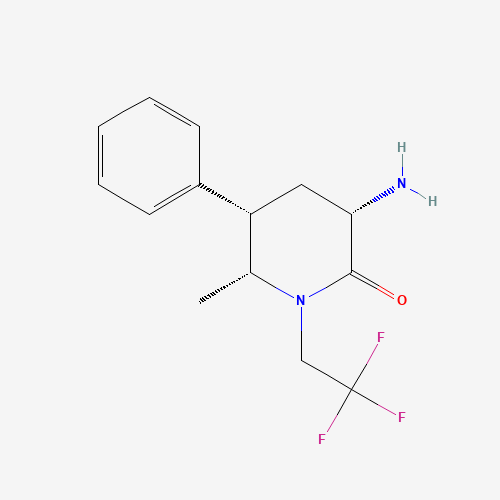
Product Information
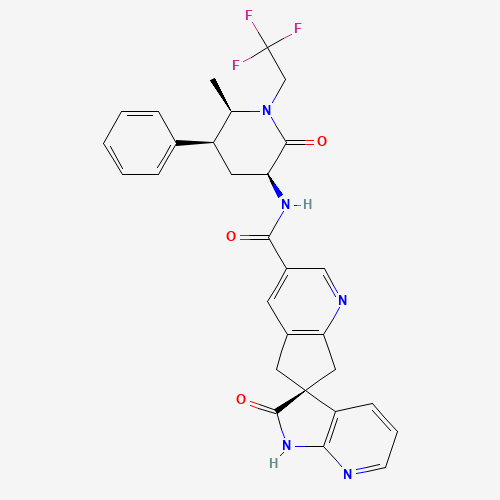
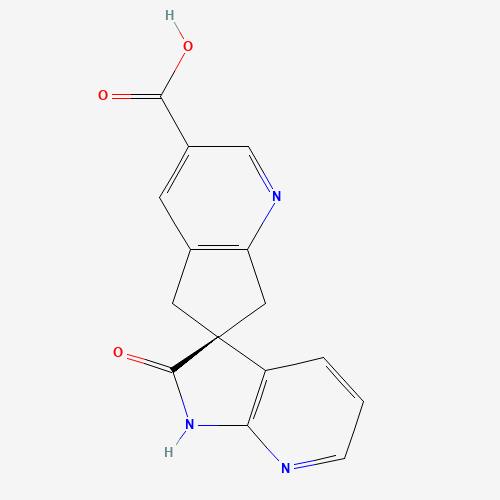
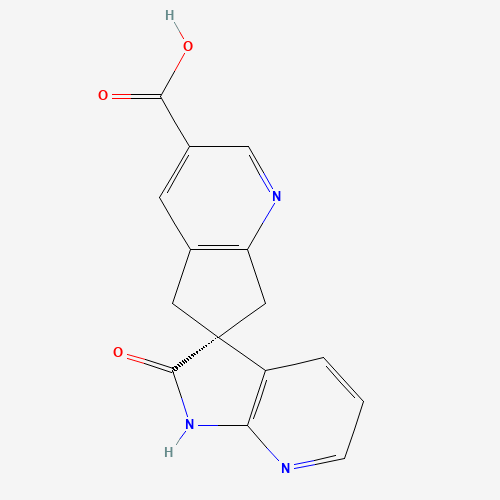
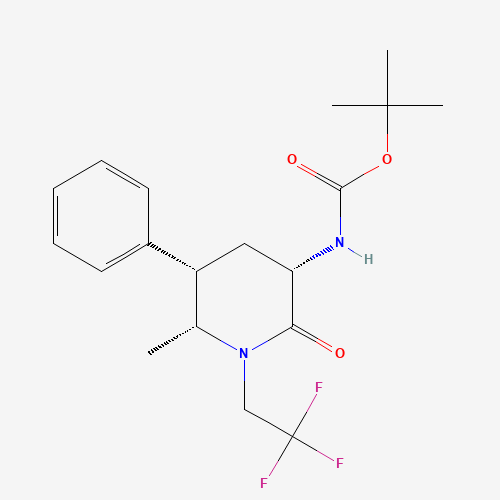
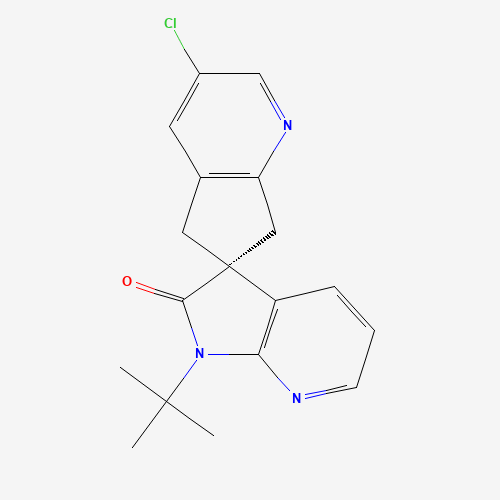
Ubrogepant Impurity 15
|
Chemical Name: Ubrogepant Impurity 15
Synonym: Spiro[6H-cyclopenta[b]pyridine-6,3'-[3H]pyrrolo[2,3-b]pyridin]-2'(1'H)-one, 3-chloro-1'-(1,1-dimethylethyl)-5,7-dihydro-, (3'R)-| Enter Batch Number | |||