Product Information
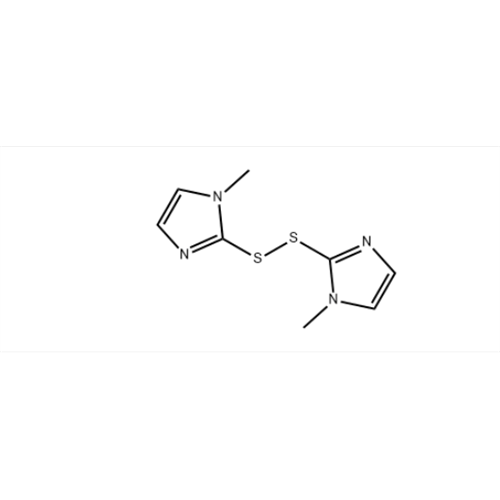
Methimazole Dimer Impurity
|
Chemical Name: Methimazole Dimer Impurity
Synonym: 1H-Imidazole, 2,2′-dithiobis[1-methyl; 2,2′-Dithiobis[1-methyl-1H-imidazole]; Bis(1-methylimidazol-2-yl) disulfide;| Enter Batch Number | |||


