Product Information
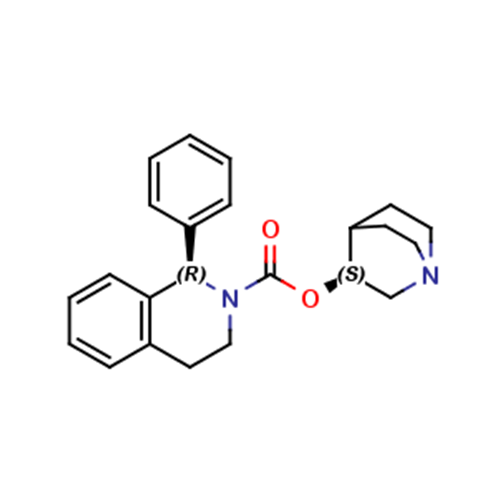
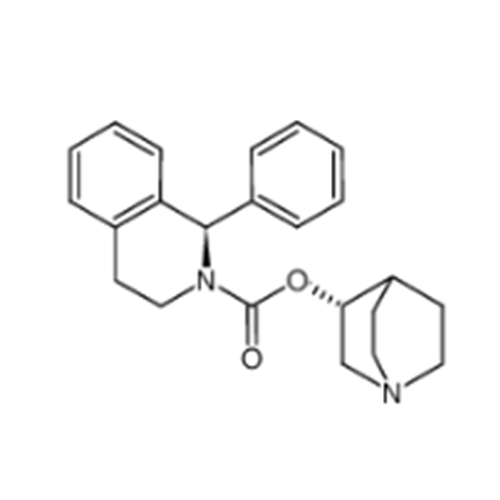
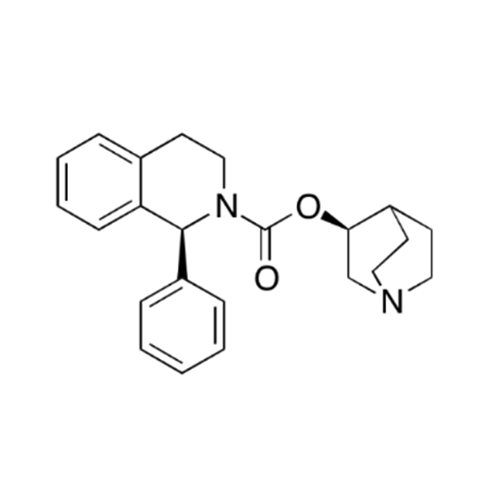
Solifenacin EP Impurity E
|
Chemical Name: Solifenacin EP Impurity E
Synonym: 3(R)-Quinuclidinol; (3R)-1-Azabicyclo[2.2.2]octan-3-ol; R-(-)-Azabicyclo[2.2.2]octane-3-ol;(R)-3-Quinuclidol; (R)-3-Hydroxyquinuclidine; (3R)-Quinuclidin-3-ol| Enter Batch Number | |||