Product Information
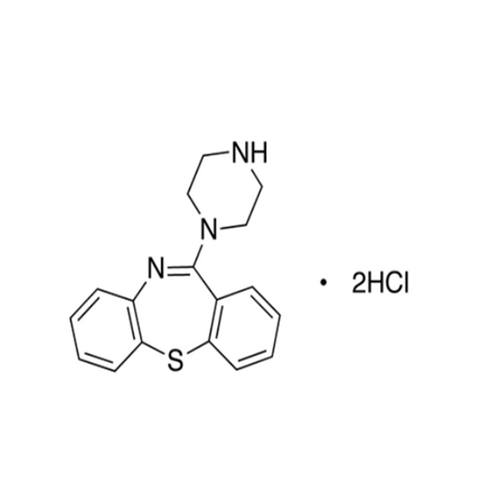
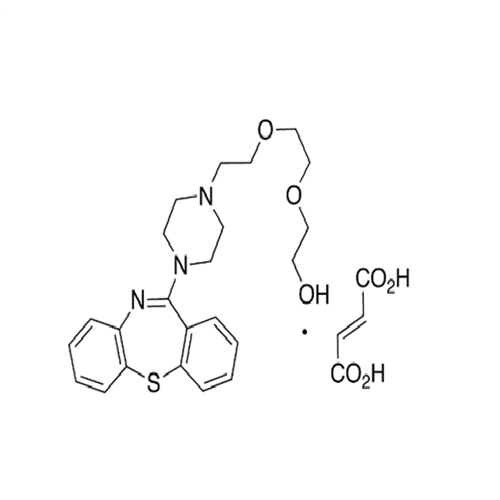
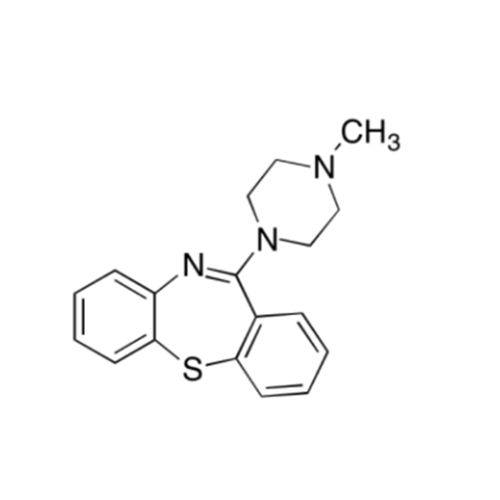
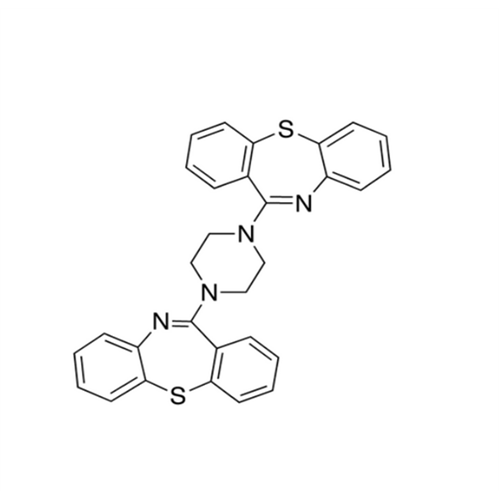
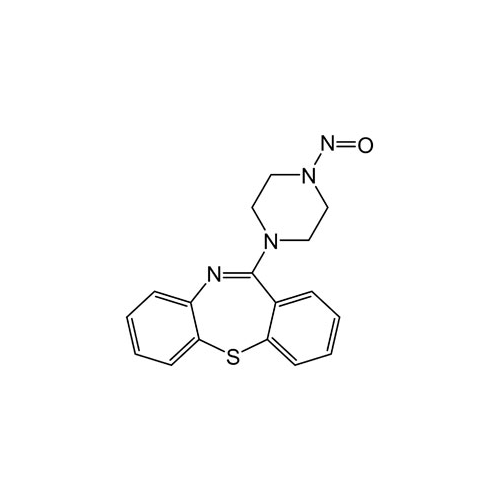
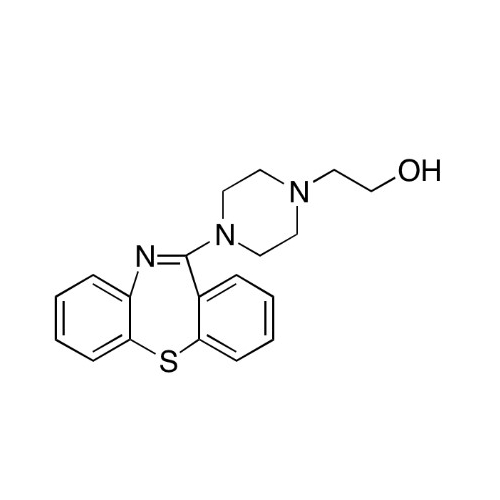
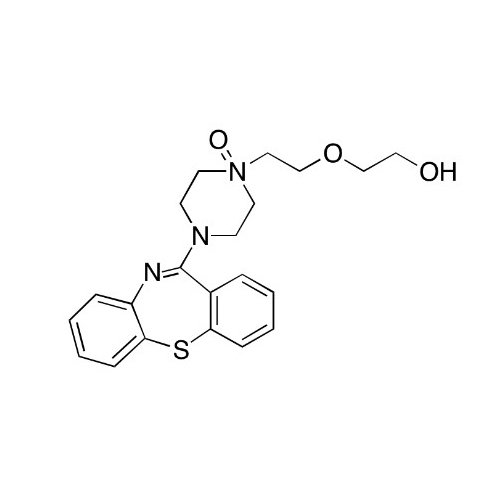
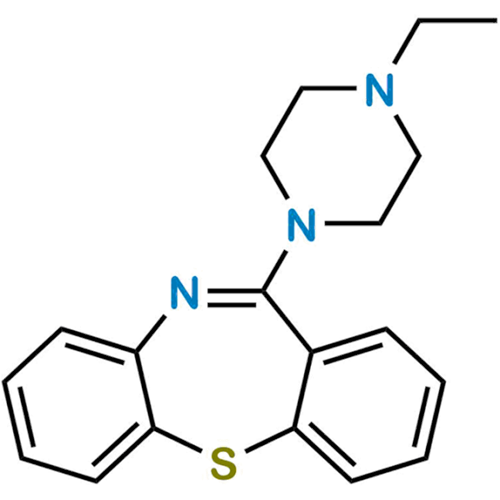
Quetiapine EP Impurity J
|
Chemical Name: Quetiapine EP Impurity J
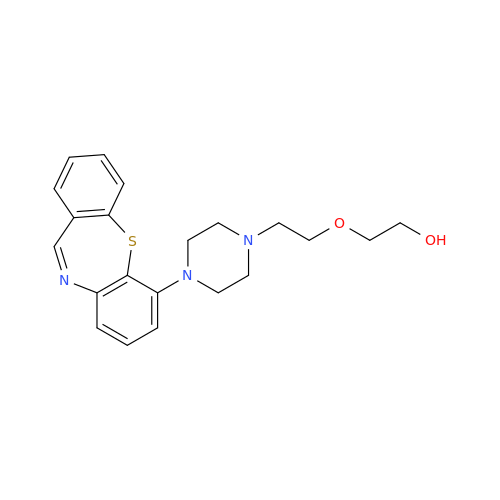
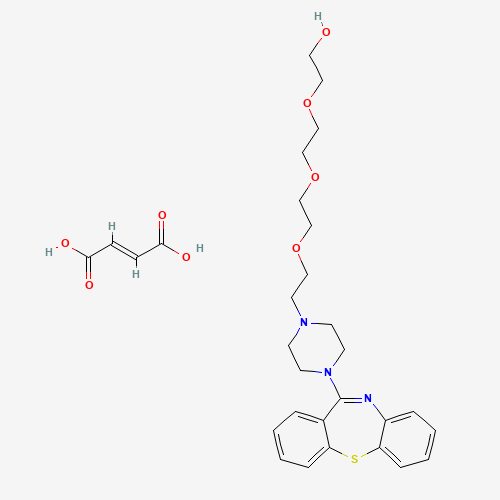
Synonym: Quetiapine tetraethylene glycol Analog; 2-[2-[2-[2-(4-Dibenzo[b,f][1,4]thiazepin-11-yl-1-piperazinyl)ethoxy]ethoxy]ethoxy]-ethanol Fumarate| Enter Batch Number | |||