Product Information
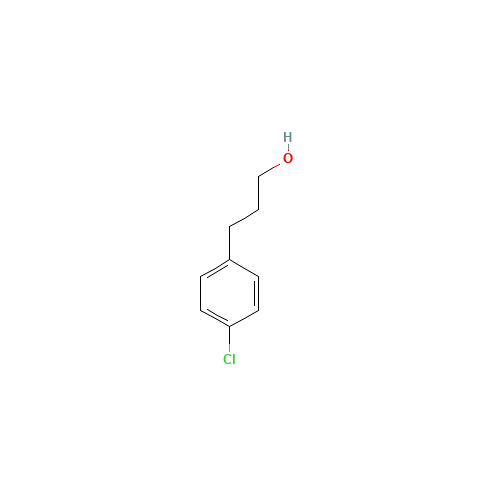
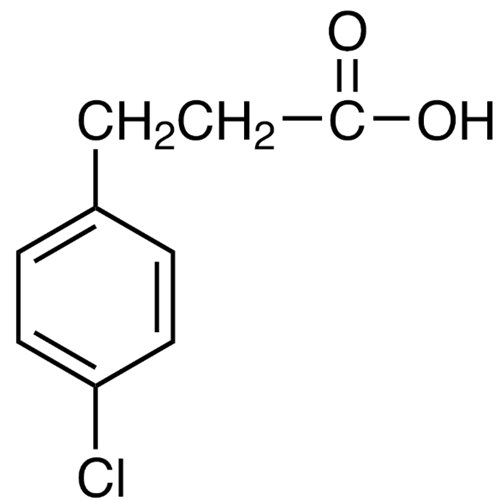
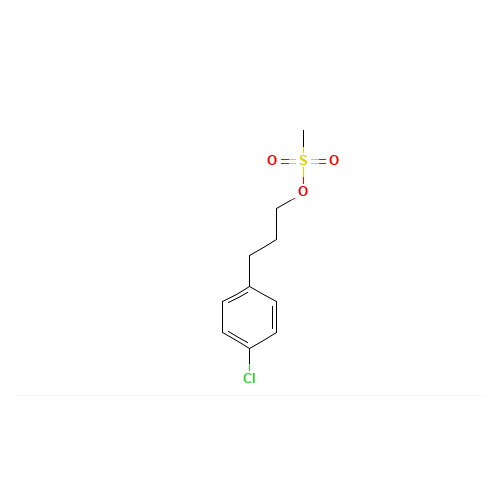
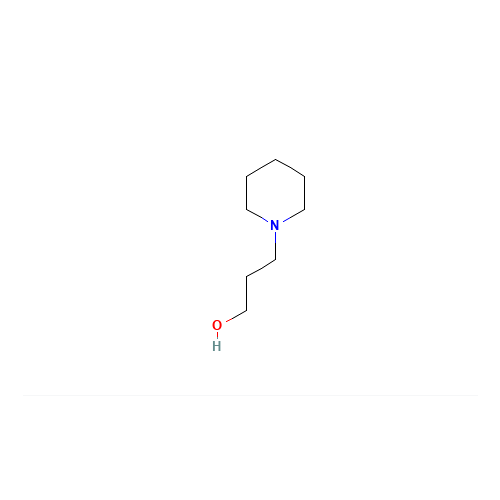
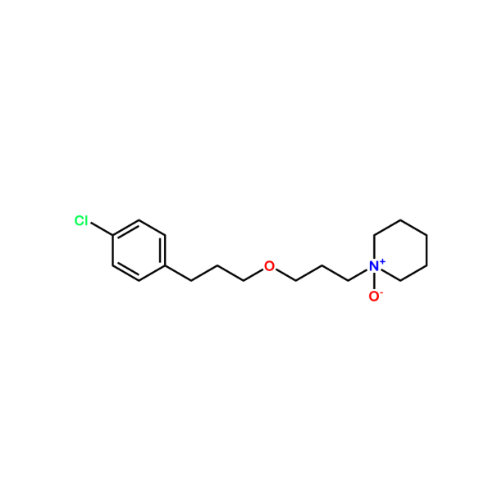
Pitolisant N-Oxide Impurity
|
Chemical Name: Pitolisant N-Oxide Impurity
Synonym: Pitolisant N-Oxide Impurity (PTS)| Enter Batch Number | |||