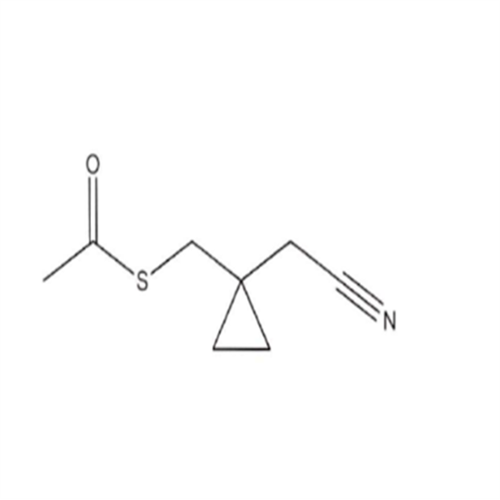
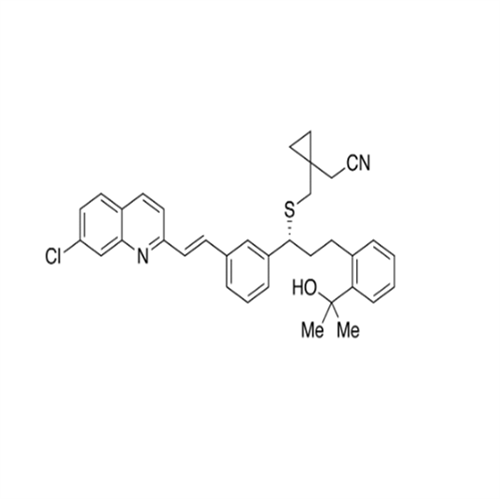
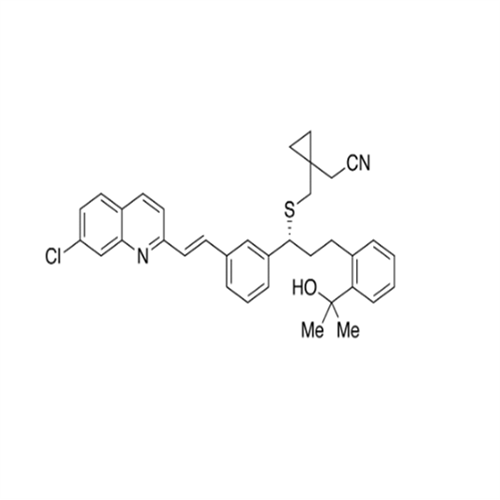
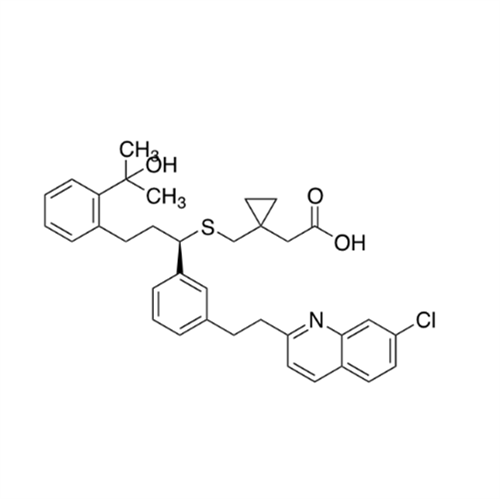
Product Information
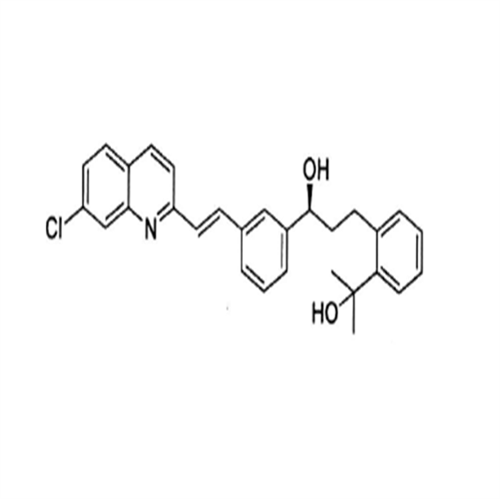
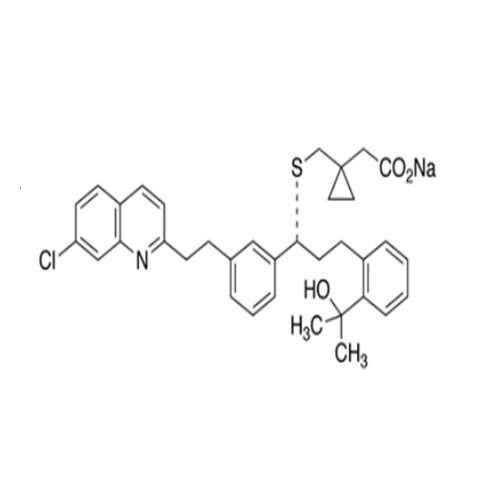
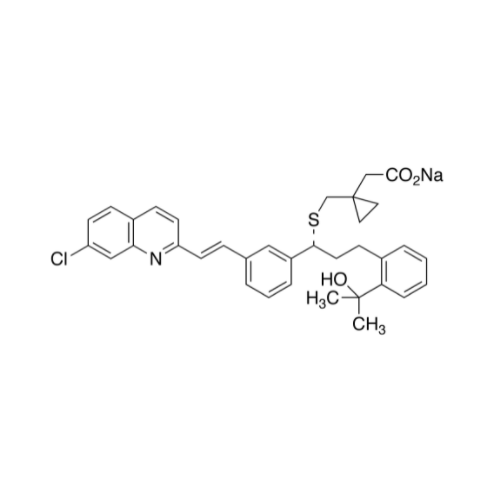
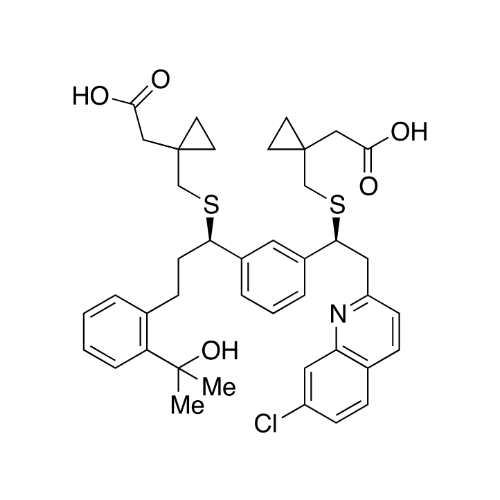
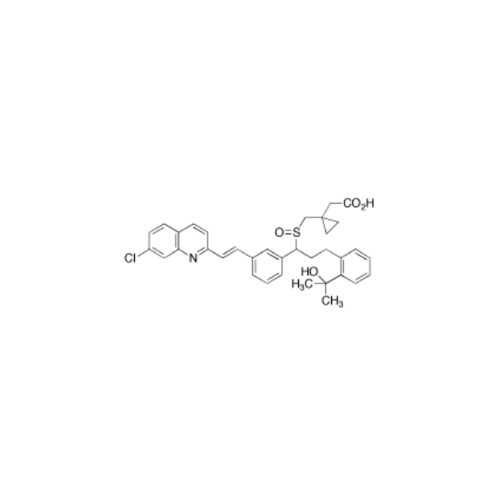
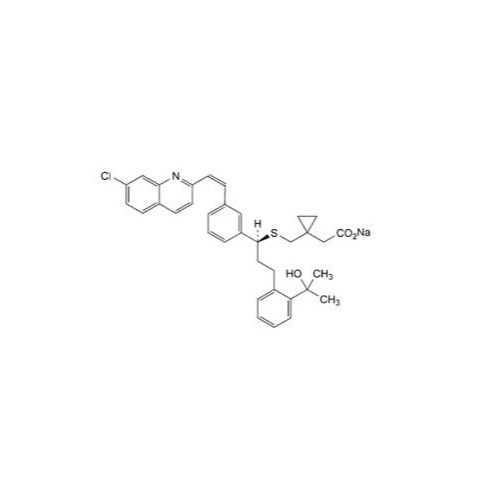
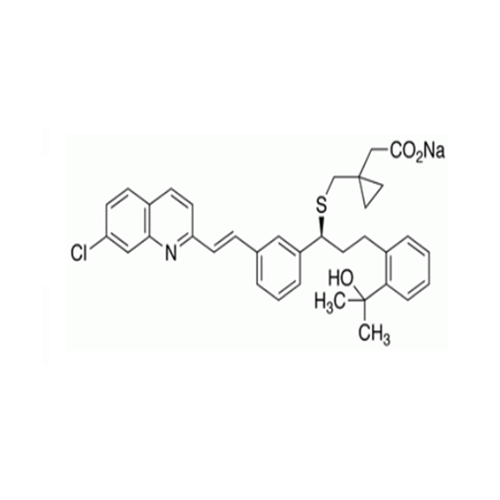
Montelukast Sodium EP Impurity A
|
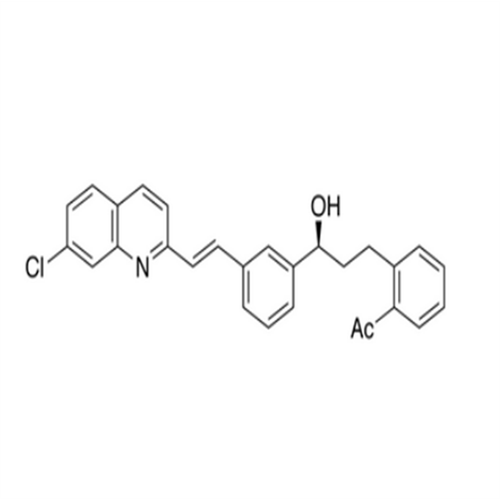
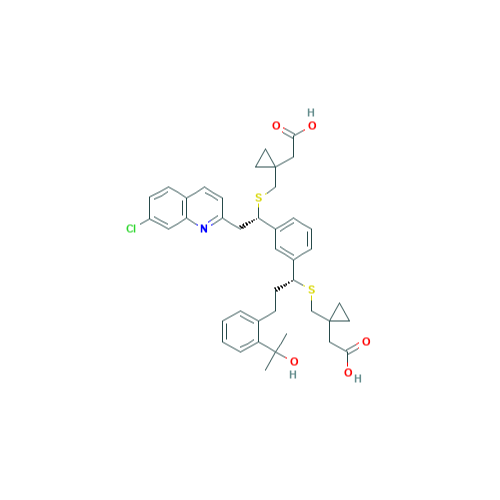
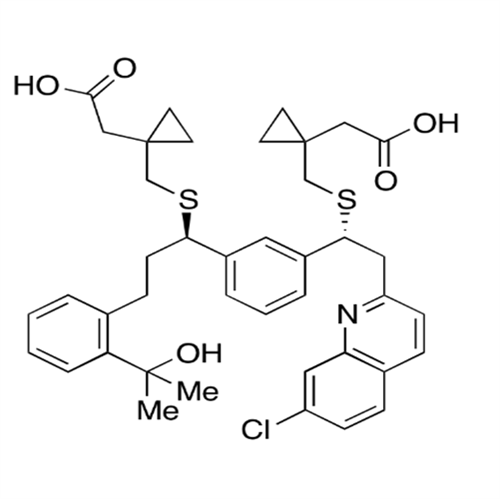
Chemical Name: Montelukast Sodium EP Impurity A
Synonym: 1-[[[(1S)-1-[3-[(1E)-2-(7-Chloro-2-quinolinyl)ethenyl]phenyl]-3-[2-(1-hydroxy-1-methylethyl)phenyl]propyl]thio]methyl]cyclopropaneacetic Acid Sodium Salt; L 768232; Montelukast EP Impurity A| Enter Batch Number | |||



![[1-(Bromomethyl) cyclopropyl] acetonitrile [1-(Bromomethyl) cyclopropyl] acetonitrile](/uploads/product-details/mon004-535.png)