Product Information
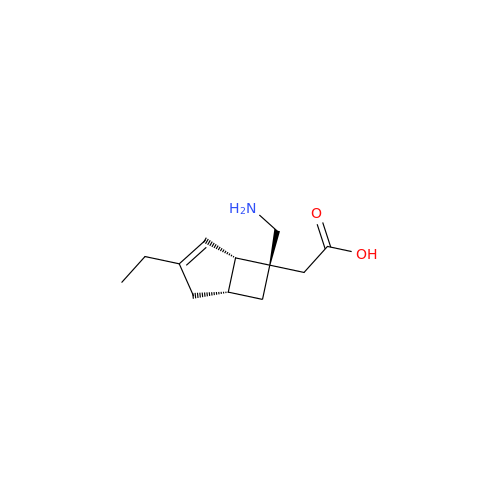
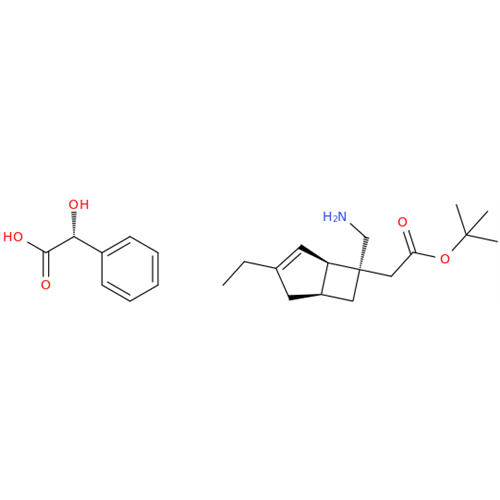
Mirogabalin Impurity 18 D-Mandelic acid
|
Chemical Name: Mirogabalin Impurity 18 D-Mandelic acid
Synonym: ((1R,5S,6S)-6-Aminomethyl-3-ethylbicyclo[3.2.0]hept-3-en-6-yl)acetic acid tert-butyl Ester D-mandelate| Enter Batch Number | |||