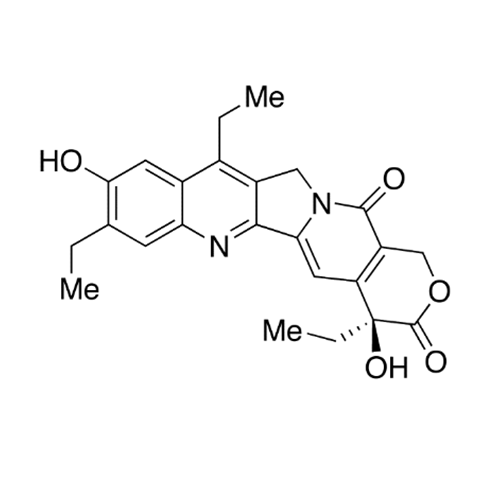
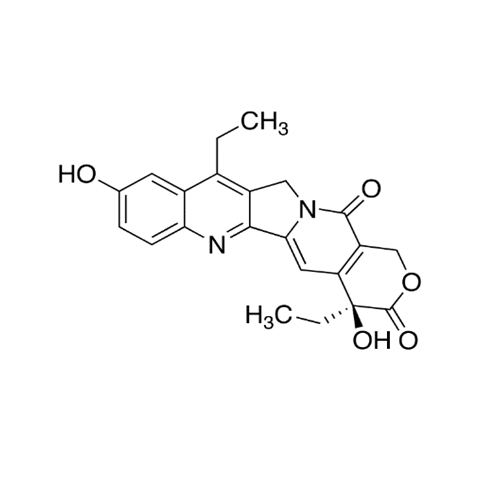
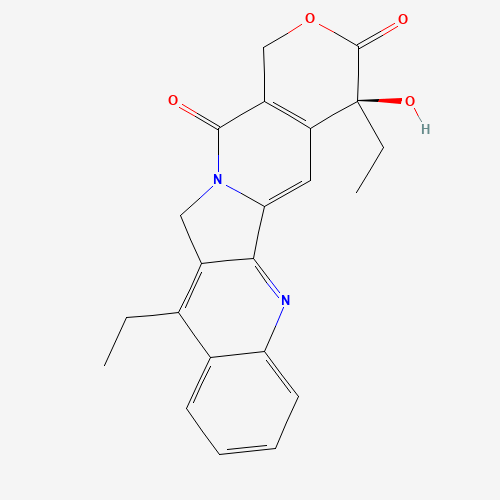
Product Information
Irinotecan IHRS
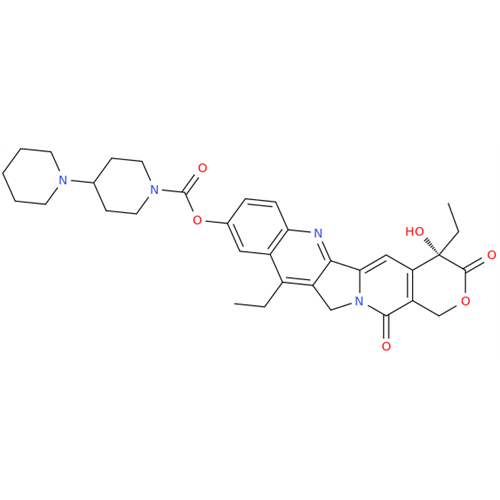
|
Chemical Name: Irinotecan IHRS
Synonym: (S)-4,11-diethyl-3,4,12,14-tetrahydro-4-hydroxy-3,14-dioxo1H-pyrano[3',4':6,7]-indolizino[1,2-b]quinolin-9-yl-[1,4'bipiperidine]-1'-carboxylate (USP)| Enter Batch Number | |||