Product Information
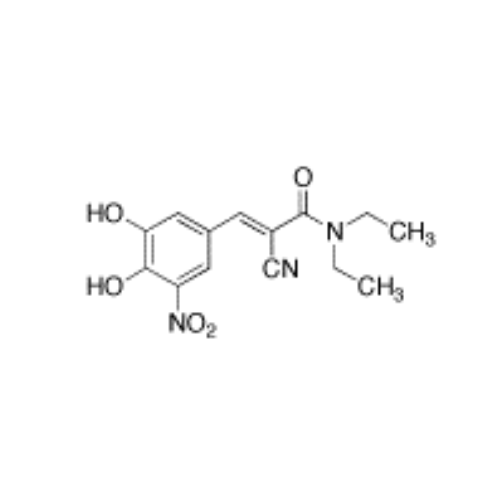
Entacapone EP Impurity H
|
Chemical Name: Entacapone EP Impurity H
Synonym: (2E)-3-(3,4-Dihydroxy-5-nitrophenyl)-2-(piperidin-1-ylcarbonyl)prop-2-ennitrile| Enter Batch Number | |||