Product Information
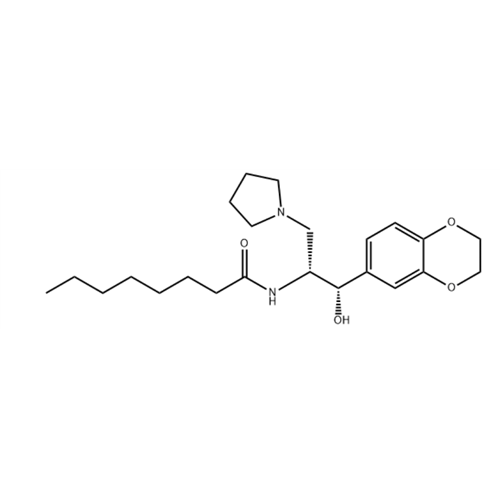
(1R,2S)-Eliglustat Diastereomer
|
Chemical Name: (1R,2S)-Eliglustat Diastereomer
Synonym: Octanamide, N-[(1R,2S)-2-(2,3-dihydro-1,4-benzodioxin-6-yl)-2-hydroxy-1-(1-pyrrolidinylmethyl)ethyl];| Enter Batch Number | |||


